Question: What is the temperature at which your calculated experimental K c value would be valid? Why is it important to state the temperature at which
What is the temperature at which your calculated experimental Kc value would be valid? Why is it important to state the temperature at which Kc is valid?
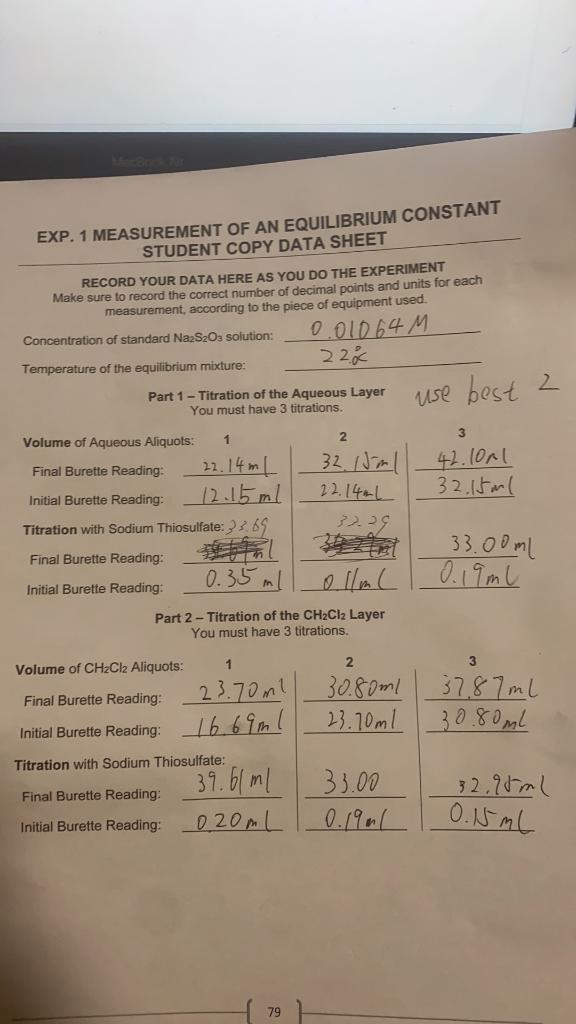
RECORD YOUR DATA HERE AS YOU DO THE EXPERIMENT Make sure to record the correct number of decimal points and units for each measurement, according to the piece of equipment used. Concentration of standard Na2S2O3 solution: Temperature of the equilibrium mixture: Part 1 - Titration of the Aqueous Layer You must have 3 titrations. Part 2 - Titration of the CH2Cl2 Layer You must have 3 titrations
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


