Question: what the answer for this problem Consider the following experimental data: Derive the rate law with the value of k for the reaction: S2O82(aq)+3I(aq)2SO42(aq)+I3(aq)
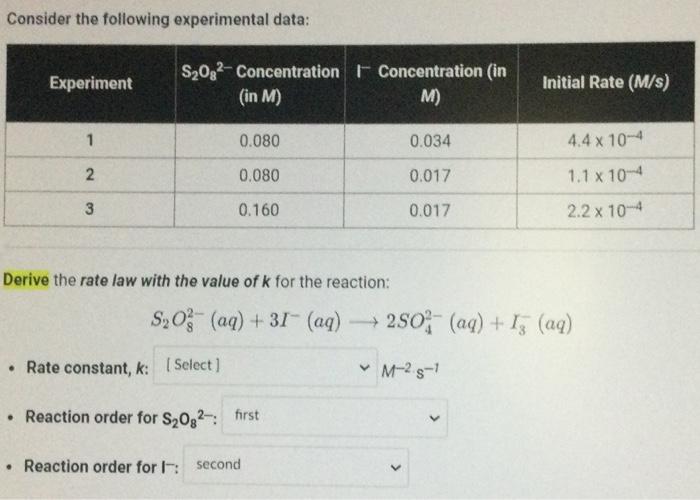
Consider the following experimental data: Derive the rate law with the value of k for the reaction: S2O82(aq)+3I(aq)2SO42(aq)+I3(aq)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


