Question: what values from question 1 should I use in my calculation for question 2? also where does the value for time go in that calculation?
 what values from question 1 should I use in my calculation for question 2? also where does the value for time go in that calculation?
what values from question 1 should I use in my calculation for question 2? also where does the value for time go in that calculation?
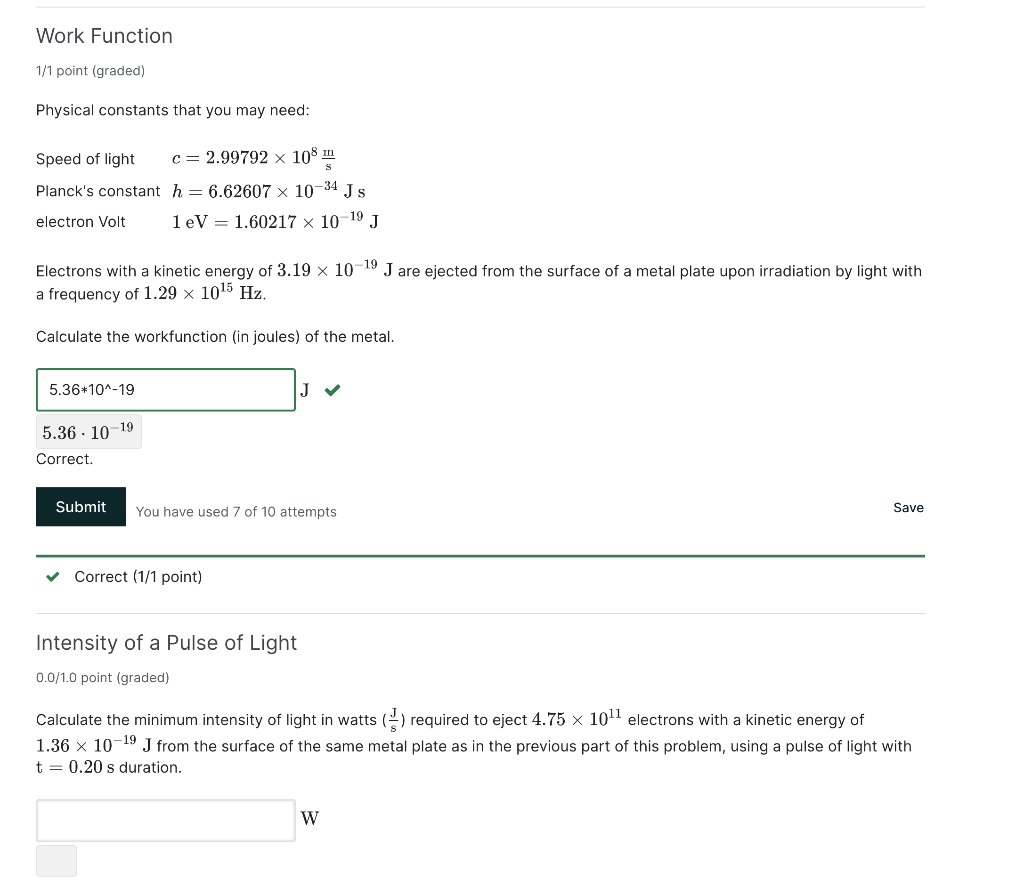
Work Function 1/1 point (graded) Physical constants that you may need: Speed of light c=2.99792108smI Planck's constant h=6.626071034Js electron Volt 1eV=1.602171019J Electrons with a kinetic energy of 3.191019J are ejected from the surface of a metal plate upon irradiation by light with a frequency of 1.291015Hz. Calculate the workfunction (in joules) of the metal. 5.361019 Correct. You have used 7 of 10 attempts Save Correct ( 1/1 point) Intensity of a Pulse of Light 0.0/1.0 point (graded) Calculate the minimum intensity of light in watts (sJ) required to eject 4.751011 electrons with a kinetic energy of 1.361019J from the surface of the same metal plate as in the previous part of this problem, using a pulse of light with t=0.20s duration. W
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


