Question: When two atoms form a single covalent bond, two What types of orbital overlap occur in cumulene? orbitals (one from each atom) overlap such that
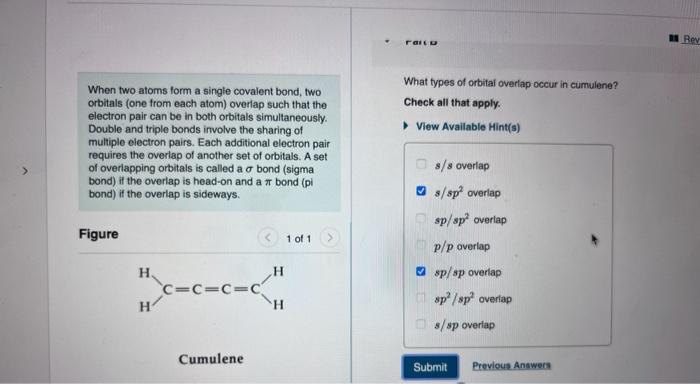
When two atoms form a single covalent bond, two What types of orbital overlap occur in cumulene? orbitals (one from each atom) overlap such that the Check all that apply. electron pair can be in both orbitals simultaneously. Double and triple bonds involve the sharing of View Available Hint(s) multiple electron pairs. Each additional electron pair requires the overlap of another set of orbitals. A set of overlapping orbitals is called a bond (sigma bond) if the overlap is head-on and a bond (pi bond) if the overlap is sideways. Figure s/s overlap D s/sp2 overlap sp/sp2 overlap p/p overlap sp/sp overlap sp2/sp2 overlap s/sp overlap Cumulene Submit Prevlous Answers
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


