Question: where a is a constant. a. Determine the relationship between the a and b parameters in this equation of state and the critical temperature and

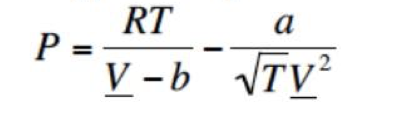
where a is a constant. a. Determine the relationship between the a and b parameters in this equation of state and the critical temperature and pressure of the fluid. b. Obtain an expression for the constant-volume heat capacity as a function of temperature, volume, the critical temperature and pressure, and the ideal heat capacity. c. The constant-volume heat capacities of real fluids diverge as the critical point is approached. Does the constant-volume heat capacity obtained from this equation of state diverge as the critical point is approached? P=VbRTTV2a where a is a constant. a. Determine the relationship between the a and b parameters in this equation of state and the critical temperature and pressure of the fluid. b. Obtain an expression for the constant-volume heat capacity as a function of temperature, volume, the critical temperature and pressure, and the ideal heat capacity. c. The constant-volume heat capacities of real fluids diverge as the critical point is approached. Does the constant-volume heat capacity obtained from this equation of state diverge as the critical point is approached? P=VbRTTV2a
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


