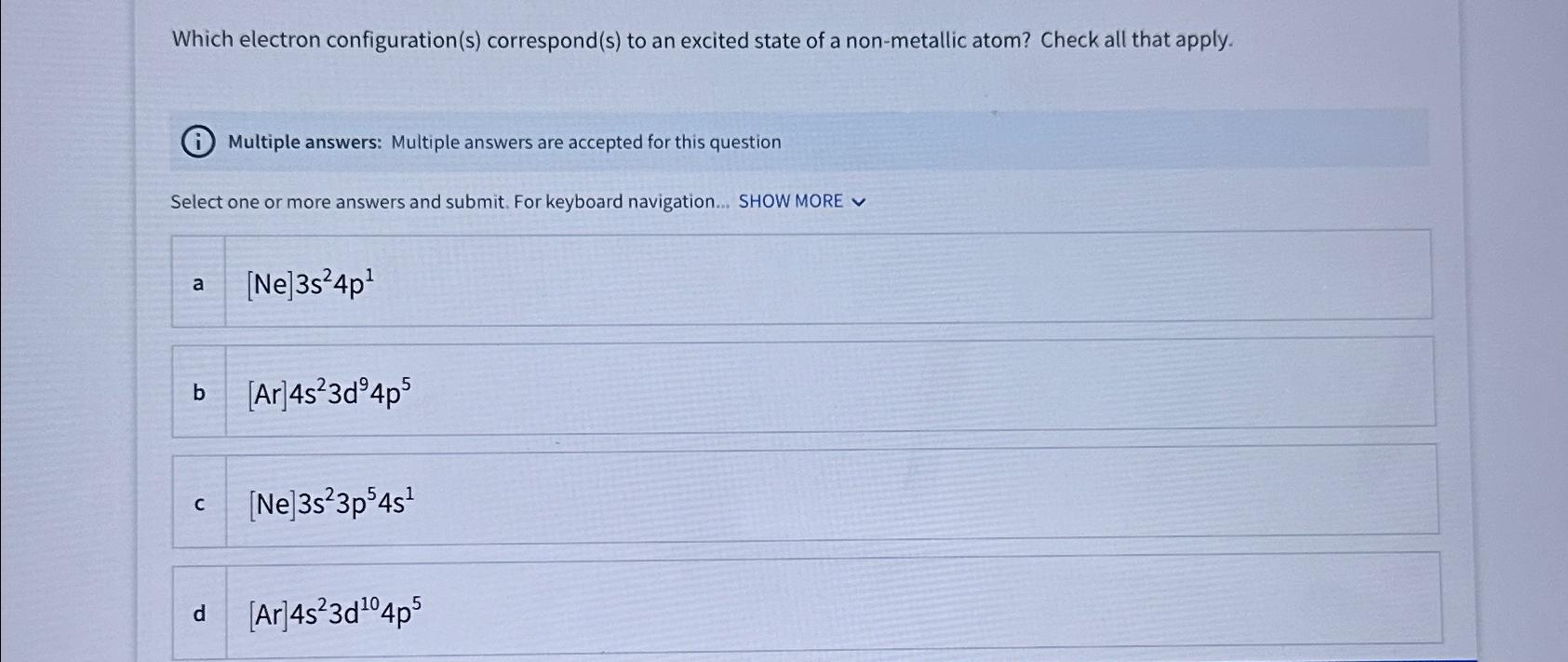
Question: Which electron configuration ( s ) correspond ( s ) to an excited state of a non - metallic atom? Check all that apply. Multiple
Which electron configurations corresponds to an excited state of a nonmetallic atom? Check all that apply.
Multiple answers: Multiple answers are accepted for this question
Select one or more answers and submit. For keyboard navigation... SHOW MORE
a
b
c
d
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


