Question: Which has a larger ionic radius between Mg2+ or 02 and why. i. Mg2+ has the larger ionic radius ii. O has the larger ionic
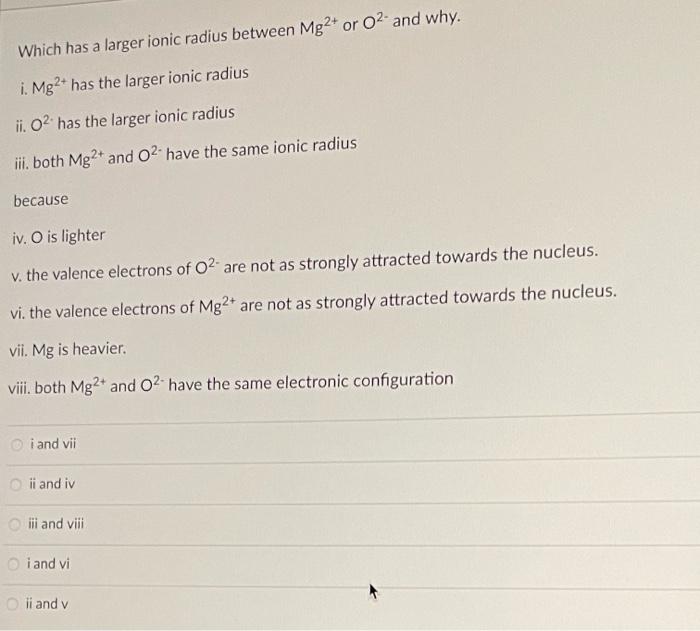
Which has a larger ionic radius between Mg2+ or 02 and why. i. Mg2+ has the larger ionic radius ii. O has the larger ionic radius iii. both Mg2+ and O2. have the same ionic radius because iv. O is lighter v. the valence electrons of O2 are not as strongly attracted towards the nucleus. vi. the valence electrons of Mg2+ are not as strongly attracted towards the nucleus. vii. Mg is heavier. vii, both Mg2+ and O2 have the same electronic configuration i and vii ii and iv iii and viii i and vi Oli and v
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


