Question: Which is a correct Lewis structure for carbonic acid, H2CO3? :o-H o=c :o=H :o-H :o-H :0=c :o=c 6H o=H None are correct :0-H :o=c
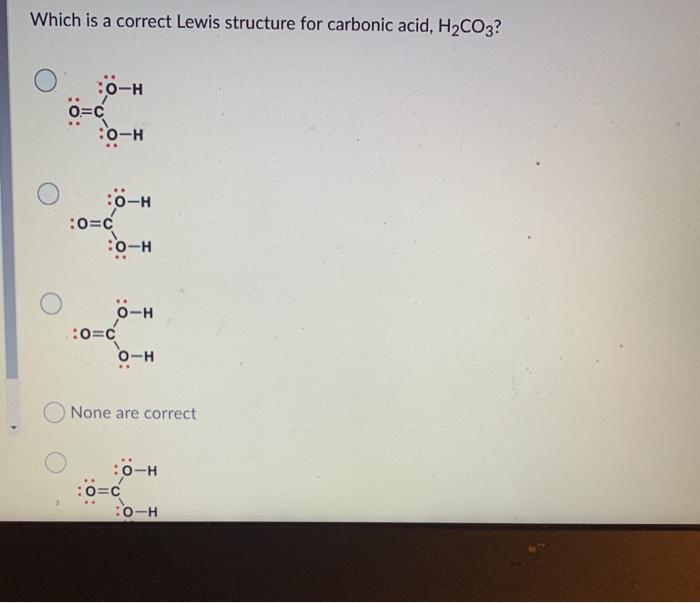
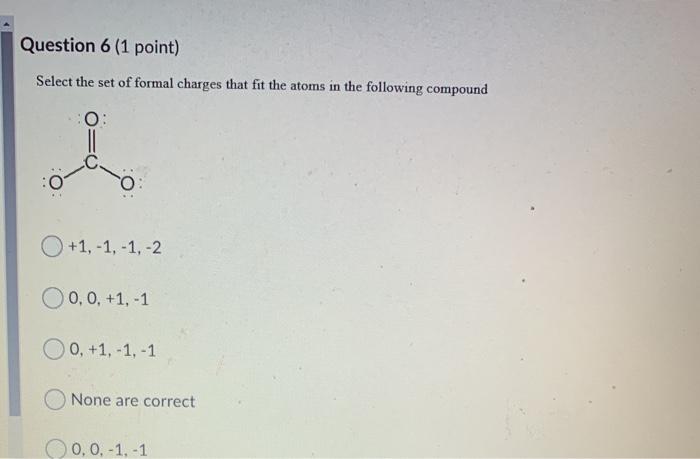

Which is a correct Lewis structure for carbonic acid, H2CO3? :o-H o=c :o=H :o-H :o-H :0=c :o=c 6H o=H None are correct :0-H :o=c :0-H Question 6 (1 point) Select the set of formal charges that fit the atoms in the following compound O: +1,-1,-1, -2 0, 0, +1, -1 0, +1,-1,-1 None are correct 0, 0,-1,-1 Name the following molecule. To minimize confusion, I would recommend you attempting the naming BEFORE looking at the given options. CH3 H3C-CH CH3 H3C-CH-CH-CH-C-CH3 HC-CH3 O2,3-diethyl-2,5-dimethylhexane None are correct 4,5-diethyl-2,5-dimethylhexane 4-ethyl-2,5,5-trimethylheptane 4-ethyl-3,3,6-trimethylheptane
Step by Step Solution
3.42 Rating (146 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


