Question: with drawing please 2) Acetone is being absorbed from air using water as the solvent. Operation is at 1atm. And at 20C. The total flow
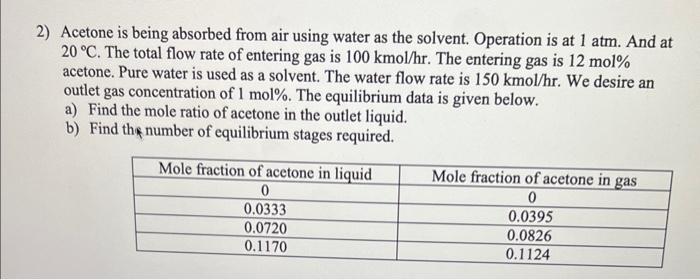
2) Acetone is being absorbed from air using water as the solvent. Operation is at 1atm. And at 20C. The total flow rate of entering gas is 100kmol/hr. The entering gas is 12mol% acetone. Pure water is used as a solvent. The water flow rate is 150kmol/hr. We desire an outlet gas concentration of 1mol%. The equilibrium data is given below. a) Find the mole ratio of acetone in the outlet liquid. b) Find the number of equilibrium stages required
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


