Question: with polymath report snd plot please 2. (3 points) The elementary, gas-phase reversible reaction CH2 - CH + 3H2 takes place in an isothermal catalyst-packed
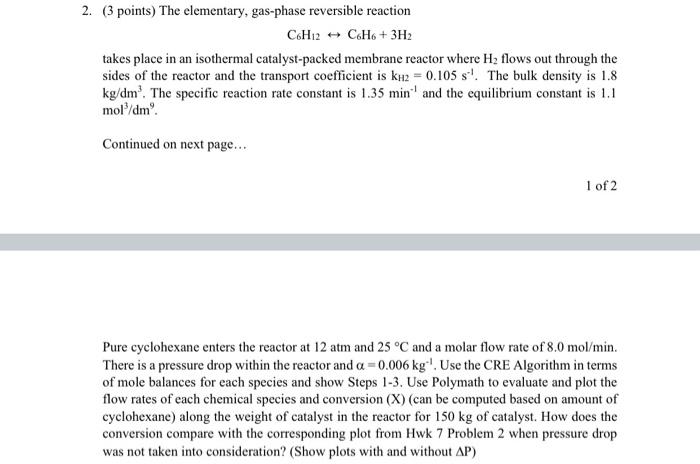
2. (3 points) The elementary, gas-phase reversible reaction CH2 - CH + 3H2 takes place in an isothermal catalyst-packed membrane reactor where H2 flows out through the sides of the reactor and the transport coefficient is ku2 = 0.105 s! The bulk density is 1.8 kg/dm? The specific reaction rate constant is 1.35 min and the equilibrium constant is 1.1 mol/dm. Continued on next page... 1 of 2 Pure cyclohexane enters the reactor at 12 atm and 25 C and a molar flow rate of 8.0 mol/min. There is a pressure drop within the reactor and a =0.006 kg '. Use the CRE Algorithm in terms of mole balances for each species and show Steps 1-3. Use Polymath to evaluate and plot the flow rates of each chemical species and conversion (X) (can be computed based on amount of cyclohexane) along the weight of catalyst in the reactor for 150 kg of catalyst. How does the conversion compare with the corresponding plot from Hwk 7 Problem 2 when pressure drop was not taken into consideration? (Show plots with and without AP)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


