Question: Working in a stable state at the beginning and inside V=2 m3 solution-containing heated-stirred tank Fi=0.1 (m3 The solution is fed at a temperature of
Working in a stable state at the beginning and inside V=2 m3 solution-containing heated-stirred tank Fi=0.1 (m3 The solution is fed at a temperature of Ti = 30 C with a flow rate of /min) and saturated steam at a flow rate of Fst=4 kg/min. is heated using Saturated steam enters the system at Tst=110 C and leaves the system at the same temperature. It comes out as a saturated liquid. The latent heat of condensation (st) of steam at the specified temperature is 2256 kJ/kg. An effective mixing is done in the boiler and due to the insulation There is no heat loss to the environment. Specific heat of solution cp=3.85 kJ/kg K and its density =1035 kg/m3 it is. with process time The dynamic model describing the temperature change is given below. a) Transfer function of the process and process block diagram create it. (10p) b) If the temperature of the feed solution rises to Ti=40 C (10 unit step increase) of the outlet temperature (T) at the new steady state find its value. (15p) Dynamic model of the process: Energy Accumulated in the System = Energy Entered into the System-Energy Out of the System
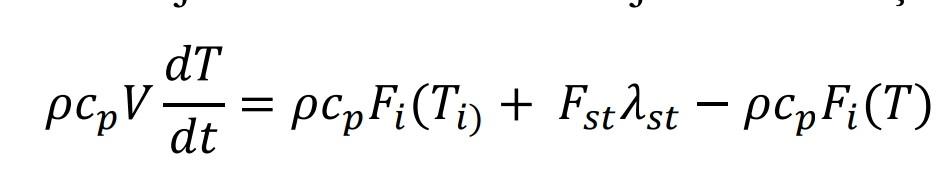
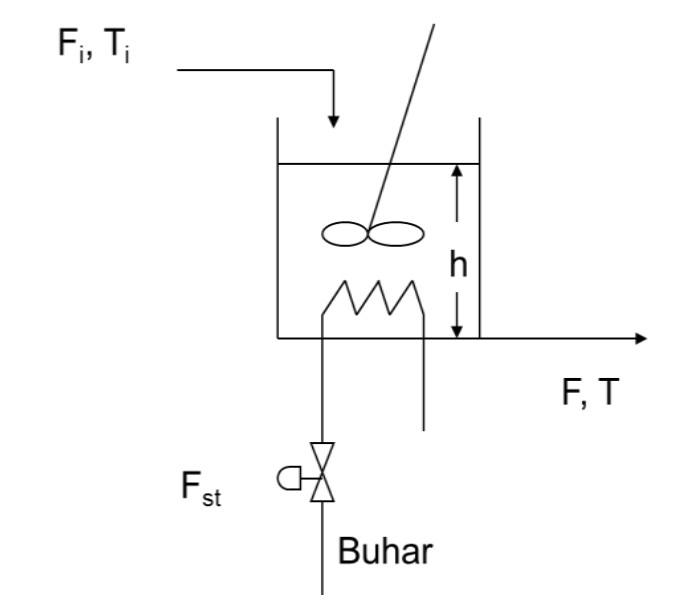
J dT = pc.. = pcpFi(Ti) + Fst 1st - pcpFi(T) dt Fi, Ti h FT Fst Buhar
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


