Question: Would ethanol (CH3CH2OH) be a suitable solvent in which to perform the following proton transfer? : NH3 + : NH3 O H. Ethanol V be
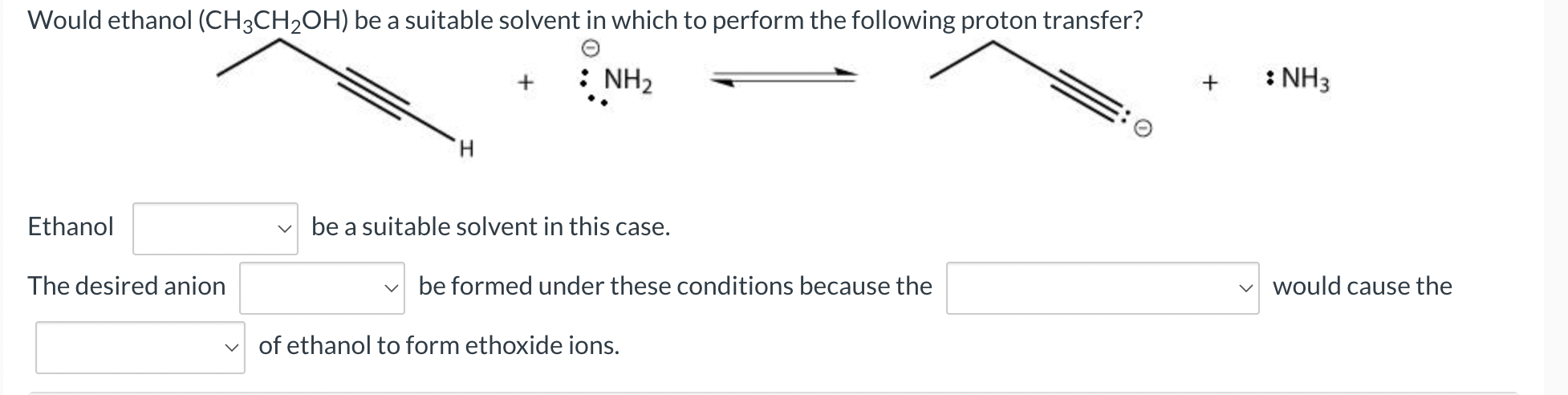
Would ethanol (CH3CH2OH) be a suitable solvent in which to perform the following proton transfer? : NH3 + : NH3 O H. Ethanol V be a suitable solvent in this case. The desired anion be formed under these conditions because the would cause the v of ethanol to form ethoxide ions
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


