Question: write Experimental Rate Constant (year -1 ) to the location marked in yellow Mentioning the source or reference from which the answer was obtained Given
write Experimental Rate Constant (year-1) to the location marked in yellow Mentioning the source or reference from which the answer was obtained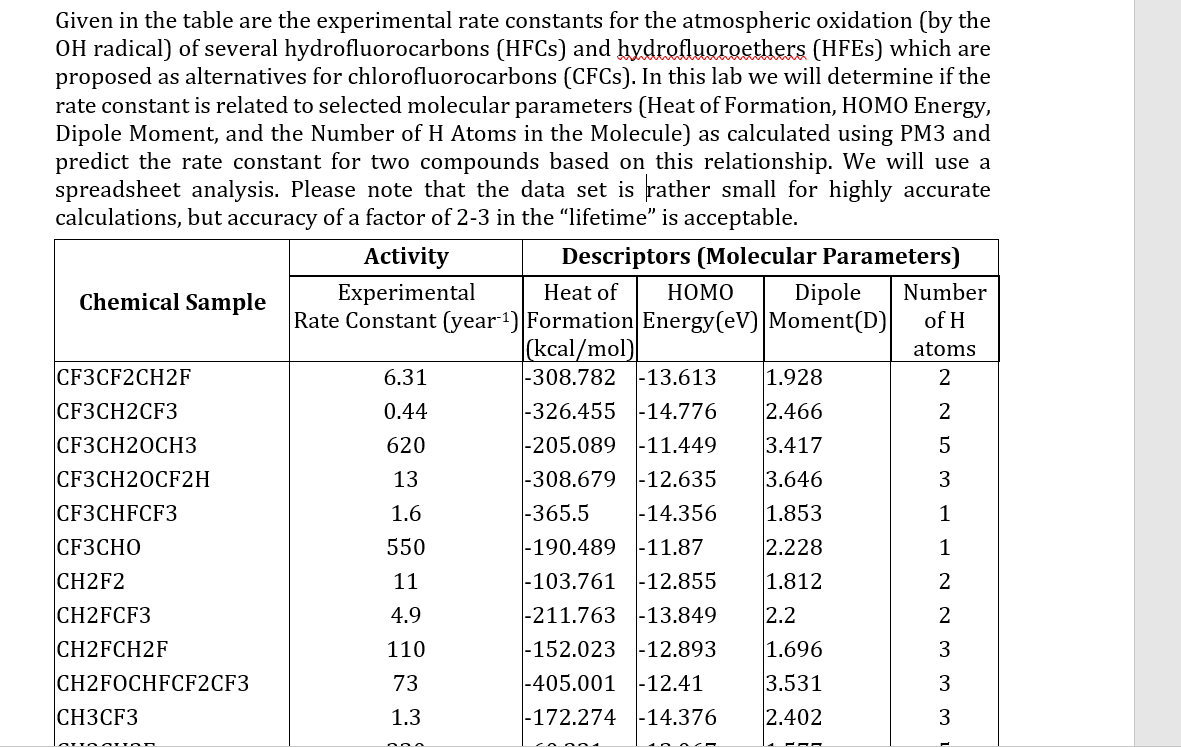
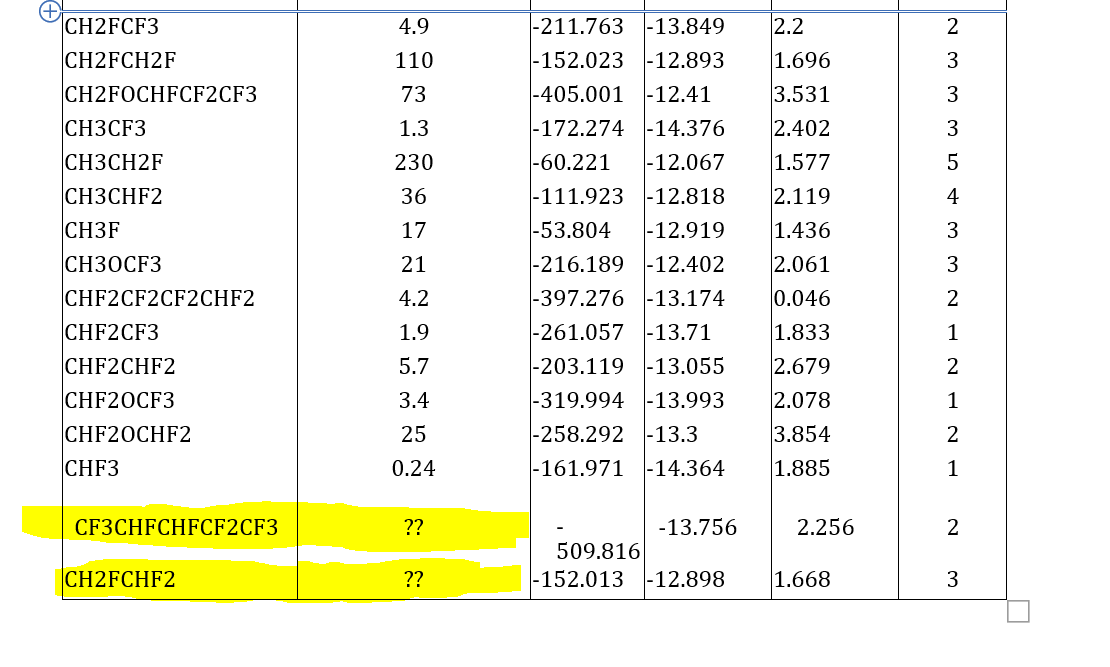
Given in the table are the experimental rate constants for the atmospheric oxidation (by the OH radical) of several hydrofluorocarbons (HFCs) and hydrofluoroethers (HFEs) which are proposed as alternatives for chlorofluorocarbons (CFCs). In this lab we will determine if the rate constant is related to selected molecular parameters (Heat of Formation, HOMO Energy, Dipole Moment, and the Number of H Atoms in the Molecule) as calculated using PM3 and predict the rate constant for two compounds based on this relationship. We will use a spreadsheet analysis. Please note that the data set is rather small for highly accurate calculations, but accuracy of a factor of 2-3 in the "lifetime" is acceptable. \begin{tabular}{|l|c|l|l|l|l|} \hline CH2FCF3 & 4.9 & 211.763 & 13.849 & 2.2 & 2 \\ CH2FCH2F & 110 & 152.023 & 12.893 & 1.696 & 3 \\ CH2FOCHFCF2CF3 & 73 & 405.001 & 12.41 & 3.531 & 3 \\ CH3CF3 & 1.3 & 172.274 & 14.376 & 2.402 & 3 \\ CH3CH2F & 230 & 60.221 & 12.067 & 1.577 & 5 \\ CH3CHF2 & 36 & 111.923 & 12.818 & 2.119 & 4 \\ CH3F & 17 & 53.804 & 12.919 & 1.436 & 3 \\ CH3OCF3 & 21 & 216.189 & 12.402 & 2.061 & 3 \\ CHF2CF2CF2CHF2 & 4.2 & 397.276 & 13.174 & 0.046 & 2 \\ CHF2CF3 & 1.9 & 261.057 & 13.71 & 1.833 & 1 \\ CHF2CHF2 & 5.7 & 203.119 & 13.055 & 2.679 & 2 \\ CHF2OCF3 & 3.4 & 319.994 & 13.993 & 2.078 & 1 \\ CHF2OCHF2 & 25 & 258.292 & 13.3 & 3.854 & 2 \\ CHF3 & 0.24 & 161.971 & 14.364 & 1.885 & 1 \\ CF3CHFCHFCF2CF3 & ?? & & 13.756 & 2.256 & 2 \\ CH2FCHF2 & ?? & 152.013 & 12.898 & 1.668 & 3 \\ \hline \end{tabular} Given in the table are the experimental rate constants for the atmospheric oxidation (by the OH radical) of several hydrofluorocarbons (HFCs) and hydrofluoroethers (HFEs) which are proposed as alternatives for chlorofluorocarbons (CFCs). In this lab we will determine if the rate constant is related to selected molecular parameters (Heat of Formation, HOMO Energy, Dipole Moment, and the Number of H Atoms in the Molecule) as calculated using PM3 and predict the rate constant for two compounds based on this relationship. We will use a spreadsheet analysis. Please note that the data set is rather small for highly accurate calculations, but accuracy of a factor of 2-3 in the "lifetime" is acceptable. \begin{tabular}{|l|c|l|l|l|l|} \hline CH2FCF3 & 4.9 & 211.763 & 13.849 & 2.2 & 2 \\ CH2FCH2F & 110 & 152.023 & 12.893 & 1.696 & 3 \\ CH2FOCHFCF2CF3 & 73 & 405.001 & 12.41 & 3.531 & 3 \\ CH3CF3 & 1.3 & 172.274 & 14.376 & 2.402 & 3 \\ CH3CH2F & 230 & 60.221 & 12.067 & 1.577 & 5 \\ CH3CHF2 & 36 & 111.923 & 12.818 & 2.119 & 4 \\ CH3F & 17 & 53.804 & 12.919 & 1.436 & 3 \\ CH3OCF3 & 21 & 216.189 & 12.402 & 2.061 & 3 \\ CHF2CF2CF2CHF2 & 4.2 & 397.276 & 13.174 & 0.046 & 2 \\ CHF2CF3 & 1.9 & 261.057 & 13.71 & 1.833 & 1 \\ CHF2CHF2 & 5.7 & 203.119 & 13.055 & 2.679 & 2 \\ CHF2OCF3 & 3.4 & 319.994 & 13.993 & 2.078 & 1 \\ CHF2OCHF2 & 25 & 258.292 & 13.3 & 3.854 & 2 \\ CHF3 & 0.24 & 161.971 & 14.364 & 1.885 & 1 \\ CF3CHFCHFCF2CF3 & ?? & & 13.756 & 2.256 & 2 \\ CH2FCHF2 & ?? & 152.013 & 12.898 & 1.668 & 3 \\ \hline \end{tabular}
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


