Question: Write mass - balance expressions for a 0 . 0 1 0 0 M solution of H C l that is in equilibrium with an
Write massbalance expressions for a solution of that is in equilibrium with an excess of solid
As shown by Equations and three equilibria are present in this solution. That is
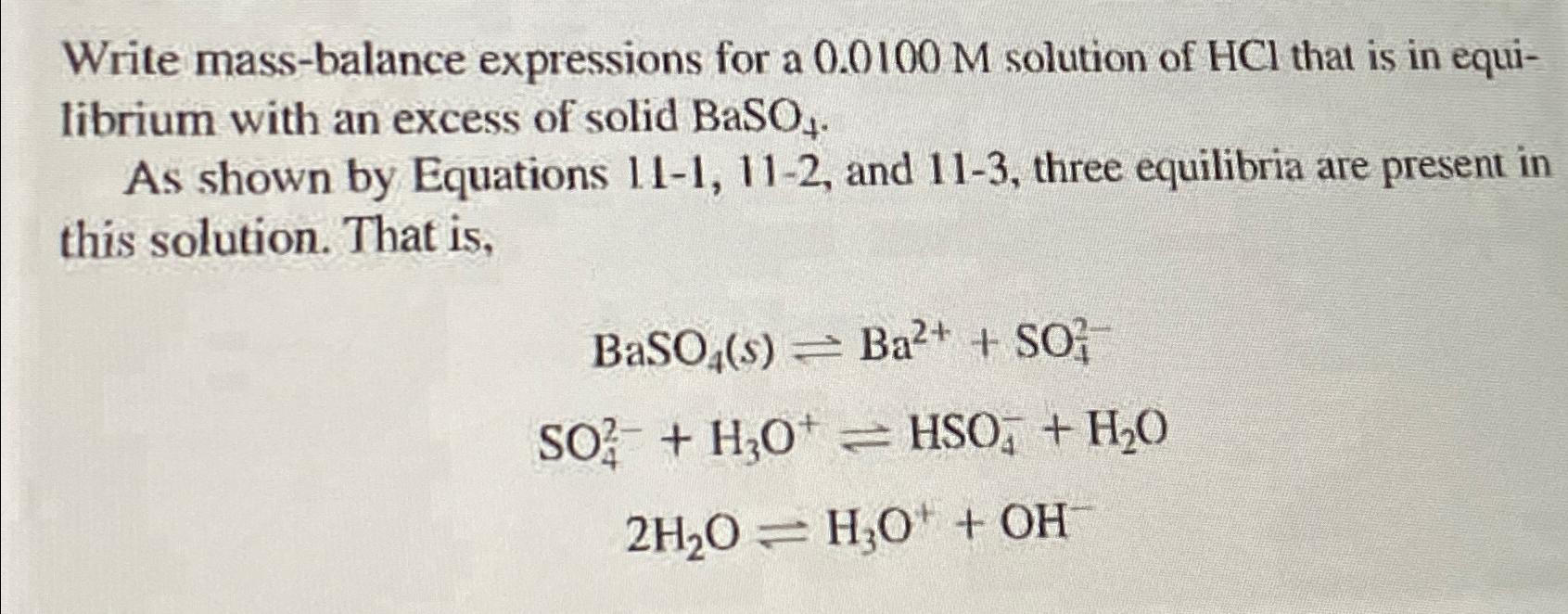
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


