Question: Write simple valence bond wave functions for the diatomic molecules B 2 and O 2 . State the bond order predicted by the simple VB
Write simple valence bond wave functions for the diatomic molecules B 2 and O 2 . State the bond order predicted by the simple VB model and compare with the LCAO predictions in Table 6.3.
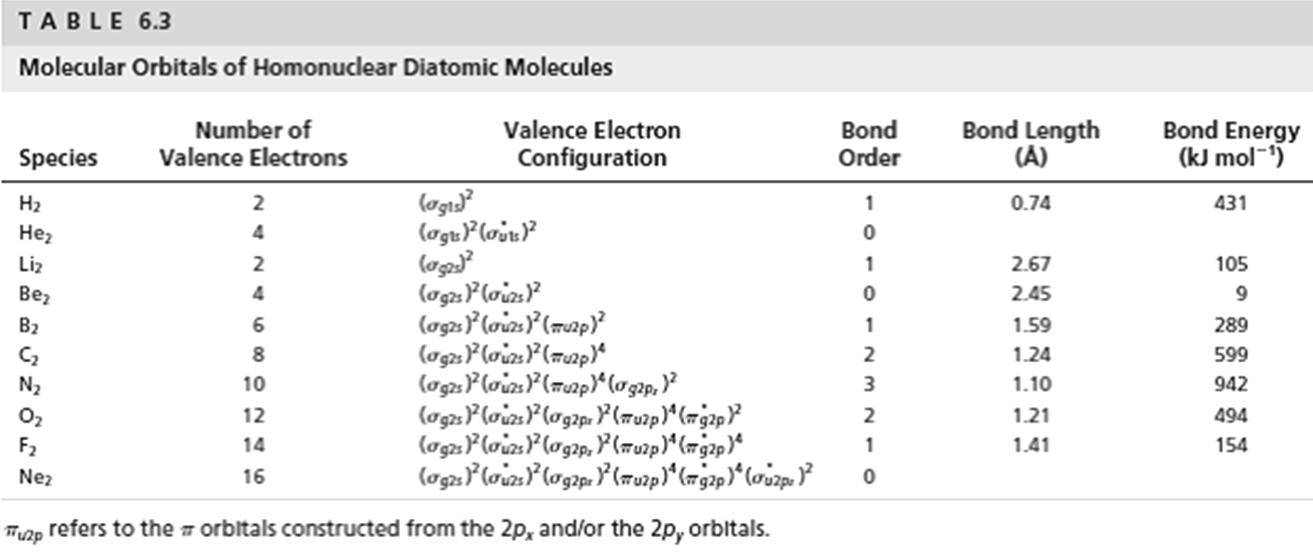
TABLE 6.3 Molecular Orbitals of Homonuclear Diatomic Molecules Number of Valence Electrons Bond Energy (kJ mol-1) Valence Electron Bond Order Bond Length (A) Species Configuration (ag3 (agtF(ojx 0.74 431 2 4 Liz 2 2.67 105 (ag2P loizs} (ag2} (oiz} (map) (ag2P loizs} (map)" Bez 4 2.45 B2 1.59 289 1.24 599 N2 10 1.10 942 O2 F2 12 2 1.21 494 (a2}loizP(og2p, P (7uzp)" (79ap)" 14 1.41 154 Nez 16 refers to the T orbitals constructed from the 2p, and/or the 2p, orbltals. Thuzp
Step by Step Solution
3.29 Rating (152 Votes )
There are 3 Steps involved in it
To write simple valence bond VB wave functions for B 2 and O 2 we focus on how the atomic orbitals o... View full answer

Get step-by-step solutions from verified subject matter experts


