Question: Write the empirical formula for at least four ionic compounds that could be formed from the following lons: NO3,MnO4,NH4+,Fe2+ Multiply or divide the following measurements.
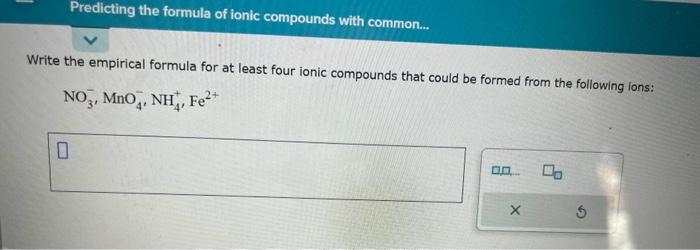
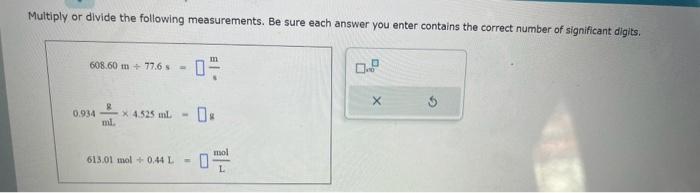

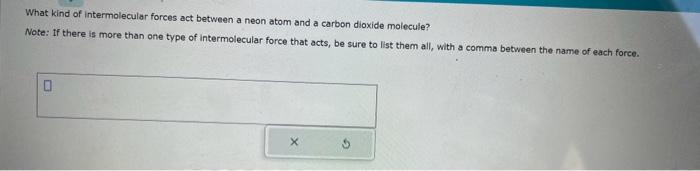
Write the empirical formula for at least four ionic compounds that could be formed from the following lons: NO3,MnO4,NH4+,Fe2+ Multiply or divide the following measurements. Be sure each answer you enter contains the correct number of significant digits. 608.60m77.6s=0.934mLg4.525mL=m613.01mol0.44L=Lmol Fill in the systematic names of the following chemical compounds. Note: for compounds containing hydrogen, you may give the common name instead. What kind of intermolecular forces act between a neon atom and a carbon dioxide molecule? Note: If there is more than one type of intermolecular force that acts, be sure to list them all, with a comma between the name of each force
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


