Question: Write the equilibrium constant expression, KC, for the following reaction: Please enter the compounds in the order given in the reaction. If either the numerator
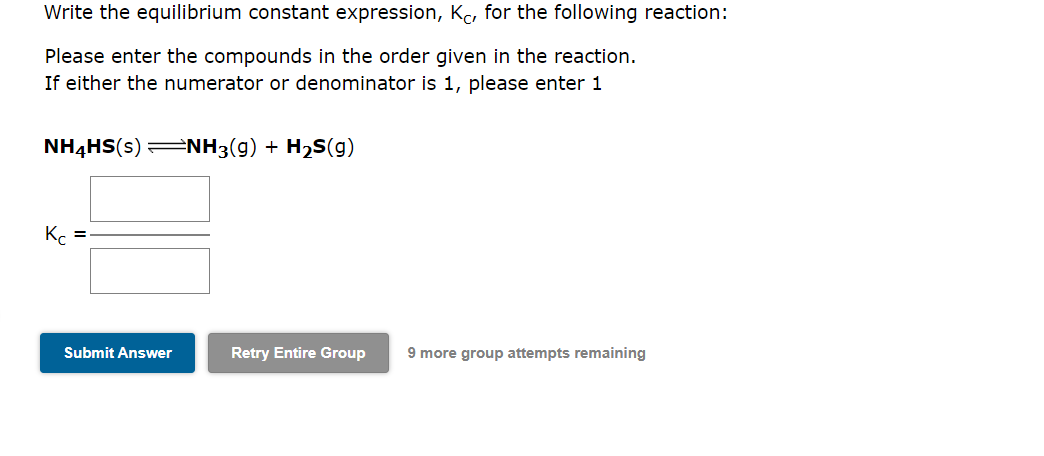


Write the equilibrium constant expression, KC, for the following reaction: Please enter the compounds in the order given in the reaction. If either the numerator or denominator is 1 , please enter 1 NH4HS(s)NH3(g)+H2S(g) KC= 9 more group attempts remaining Write the equilibrium constant expression, KC, for the following reaction: Please enter the compounds in the order given in the reaction. If either the numerator or denominator is 1 , please enter 1 NH3(g)+H2S(g)NH4HS(s) Kc= Write the expression for the equilibrium constant Kp for the following reaction. Enclose pressures in parentheses and do NOT write the chemical formula as a subscript. For example, enter (PNH3)2 as (PNH3)2. If either the numerator or denominator is 1 , please enter 1 2WO3(s)2WO2(s)+O2(g) K=
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


