Question: Write the formula for the ionic compound formed from each pair of elements. Part 1 of 2 Strontium and chlorine: Part 2 of 2 Nickel
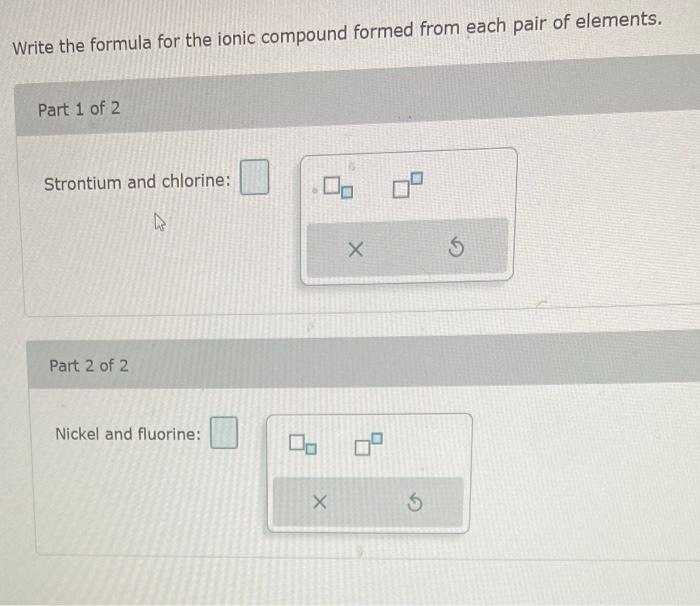
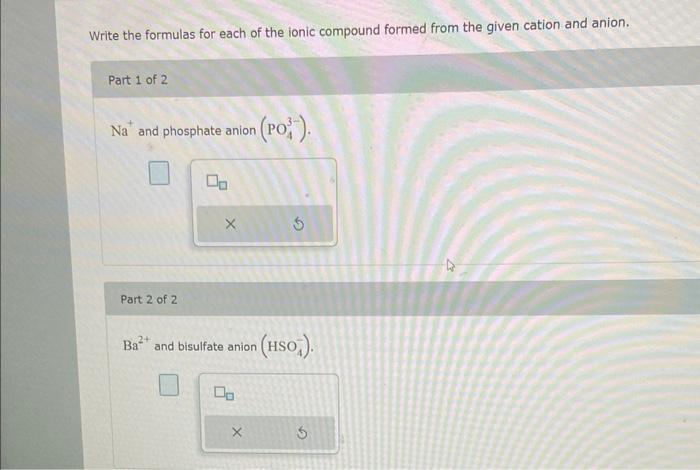
Write the formula for the ionic compound formed from each pair of elements. Part 1 of 2 Strontium and chlorine: Part 2 of 2 Nickel and fluorine: Write the formulas for each of the ionic compound formed from the given cation and anion. Part 1 of 2 Na+and phosphate anion (PO43). Part 2 of 2 Ba2+ and bisulfate anion (HSO4)
Step by Step Solution
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


