Question: Written Problem The Carnot cycle is an idealization that involves four processes as shown ( see also Figure 6 - 3 7 in textbook )
Written Problem
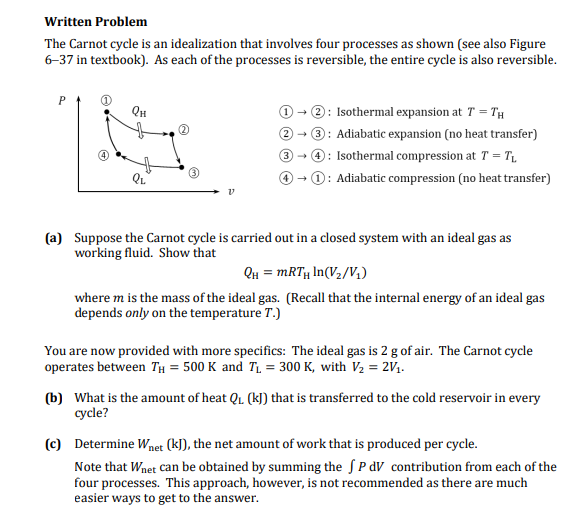
The Carnot cycle is an idealization that involves four processes as shown see also Figure
in textbook As each of the processes is reversible, the entire cycle is also reversible.
: Isothermal expansion at
: Adiabatic expansion no heat transfer
: Isothermal compression at
: Adiabatic compression no heat transfer
a Suppose the Carnot cycle is carried out in a closed system with an ideal gas as
working fluid. Show that
where is the mass of the ideal gas. Recall that the internal energy of an ideal gas
depends only on the temperature T
You are now provided with more specifics: The ideal gas is of air. The Carnot cycle
operates between and with
b What is the amount of heat that is transferred to the cold reservoir in every
cycle?
c Determine the net amount of work that is produced per cycle.
Note that can be obtained by summing the contribution from each of the
four processes. This approach, however, is not recommended as there are much
easier ways to get to the answer.
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


