Lanthanoid zirconates Ln 2 Zr 2 O 7 (Ln = La, Pr, Nd, Sm, Eu) adopt the
Question:
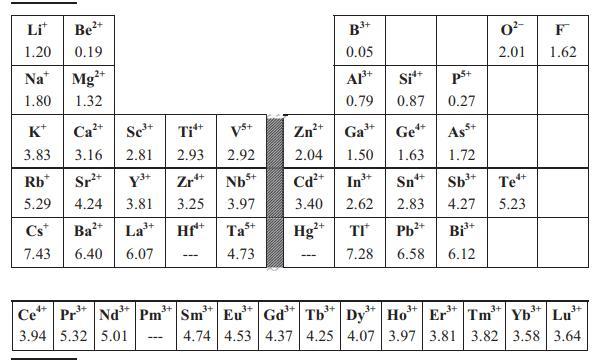
Lanthanoid zirconates Ln2Zr2O7 (Ln = La, Pr, Nd, Sm, Eu) adopt the cubic pyrochlore structure (Fd3m, Z = 8), with cubic unit-cell edge a = 10.80 Å (La), 10.69 Å (Pr), 10.67 Å (Nd), 10.59 Å (Sm), 10.55 Å (Eu). Use this information, Equation (8.13) and Table 8.2 to estimate the dielectric permittivity of these phases. Does the Clausius–Mossotti equation predict that the dielectric constant will increase or decrease asthe radius of the rare-earth ion decreases?
Equation (8.13)
![3 a -V 4 (r + 2) [CGSes : a in A, V in A] Er](https://dsd5zvtm8ll6.cloudfront.net/images/question_images/1705/9/2/1/24565ae4addc65231705921243881.jpg)
Table 8.2
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Solid State Materials Chemistry
ISBN: 9780521873253
1st Edition
Authors: Patrick M. Woodward, Pavel Karen, John S. O. Evans, Thomas Vogt
Question Posted:





