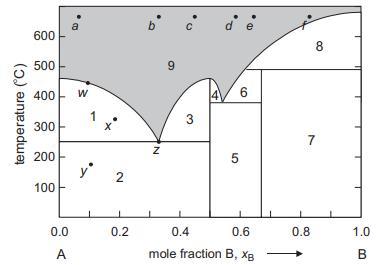
Question: Refer to the phase diagram depicted below. (a) State which four phases are stable at 100 C. (b) What is the name given to the
Refer to the phase diagram depicted below.
(a) State which four phases are stable at 100 °C.
(b) What is the name given to the horizontal line separating region 2 from 1 and 3?
(c) What are the approximate melting points of A, AB, and B?
(d) What happens if you try and melt solid AB2?
(e) State what phases are present in each of areas 1–9.
(f) Do any of the phases depicted form solid solutions?
(g) State the number of phases and degrees of freedom at points a, w, x, y, and z.
(h) Describe what happens when compositions at each of points a to f are cooled from high temperature.
(i) Estimate the relative amounts of solid and liquid when a composition at point a (xB ≈ 5) is cooled to 500 °C, 400 °C, and 300 °C.
(j) What might be observed if composition at point e is cooled rapidly?
(k) State the differences between the peritectic reactions that happen on cooling compositions at points e and f.
temperature (C) 600 500 400 300 200 100 0.0 A a W 1 y X 2 0.2 b N 9 3 6 0.4 5 0.6 mole fraction B, XB 8 7 0.8 1.0 B
Step by Step Solution
3.53 Rating (156 Votes )
There are 3 Steps involved in it
Mostly discussed in text a At 100 C A AB AB2 and B are all thermodynamically stable b Line is called the solidus c Melting points are approximately 46... View full answer

Get step-by-step solutions from verified subject matter experts


