Question:
The article €œImproved Bioequivalence Assessment of Topical Dermatological Drug Products Using Dermatopharmacokinetics€ (B. N€™Dri-Stempfer, W. Navidi, R. Guy, and A. Bunge, Pharmaceutical Research, 2009:316€“328) described a study comparing the amounts of econozole nitrate absorbed into human skin for several formulations of antifungal ointment. Both a brand name and generic drug were applied to the arms of 14 subjects, and the amounts absorbed, in μg/cm
2, were measured. Following are the results. Can you conclude that the mean amount absorbed differs between the brand name and the generic drug?
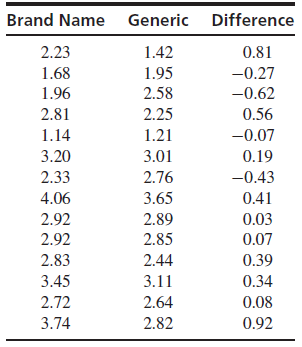
Transcribed Image Text:
Brand Name Generic Difference 2.23 1.42 0.81 1.68 1.95 -0.27 2.58 2.25 1.96 -0.62 2.81 0.56 1.14 1.21 -0.07 3.20 3.01 0.19 2.76 2.33 -0.43 4.06 3.65 0.41 0.03 2.92 2.89 2.92 2.85 0.07 2.83 2.44 0.39 3.45 0.34 3.11 2.72 2.64 0.08 3.74 2.82 0.92








