In acidic methanol, 3-oxobutanal is transformed into a compound with the molecular formula C 6 H 12
Question:
In acidic methanol, 3-oxobutanal is transformed into a compound with the molecular formula C6H12O3.
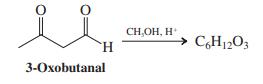
As a group, analyze the following 1H NMR and IR spectral data: 1H NMR (CCl4): δ = 2.19 (s, 3 H), 2.75 (d, 2 H), 3.38 (s, 6 H), 4.89 (t, 1 H) ppm; IR: 1715 cm-1.
Consider the chemical shifts, the splitting patterns, and the integrations of the signals in the NMR spectrum and discuss possible fragments that could give rise to the observed multiplicities. Use the IR information to assign the functional group that exists in the new molecule. Present an explanation for your structural determination, including reference to the spectral data, and suggest a detailed mechanism for the formation of the new compound.
Step by Step Answer:

Organic Chemistry structure and function
ISBN: 978-1429204941
6th edition
Authors: K. Peter C. Vollhardt, Neil E. Schore





