Question: Show the important resonance structures for these compounds. Use the curved arrow convention to show how the electrons are moved to create each new resonance
Show the important resonance structures for these compounds. Use the curved arrow convention to show how the electrons are moved to create each new resonance structure.
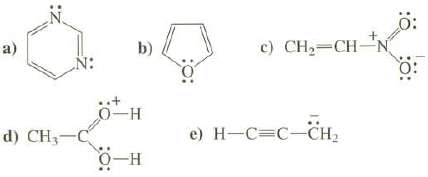
N: d) CH-C b) 0-H O-H c) CH=CH-N e) H-C=C-CH : 0:
Step by Step Solution
3.40 Rating (166 Votes )
There are 3 Steps involved in it
a The unshared electron pairs on the nitrogens are not part of the conjugated pi system b One uns... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-C (116).docx
120 KBs Word File


