Explain the following phenomenon: You have a test tube with an aqueous solution of silver nitrate as
Question:
Explain the following phenomenon: You have a test tube with an aqueous solution of silver nitrate as shown in test tube 1 below. A few drops of aqueous sodium chromate solution was added with the end result shown in test tube 2. A few drops of aqueous sodium chloride solution was then added with the end result shown in test tube 3
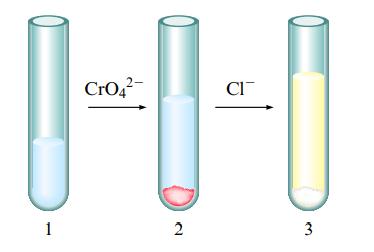
Use the Ksp values in the book to support your explanation, and include the balanced equations. Also, list the ions that are present in solution in each test tube.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





