The following plot shows the vapor pressure of various solutions of components A and B at some
Question:
The following plot shows the vapor pressure of various solutions of components A and B at some temperature.
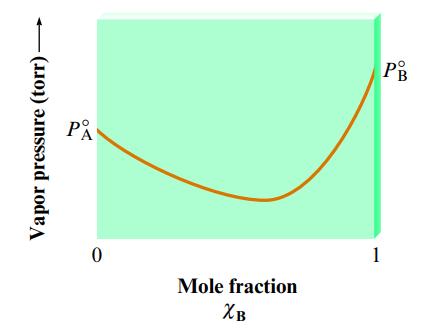
Which of the following statements is false concerning solutions of A and B?
a. The solutions exhibit negative deviations from Raoult’s law.
b. ΔHsoln for the solutions should be exothermic.
c. The intermolecular forces are stronger in solution than in either pure A or pure B.
d. Pure liquid B is more volatile than pure liquid A.
e. The solution with xB = 0.6 will have a lower boiling point than either pure A or pure B.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Chemistry
ISBN: 9781305957404
10th Edition
Authors: Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Question Posted:





