A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and
Question:
A 17.3-liter tank contains a mixture of argon, helium, and nitrogen at 298 K. The argon and helium mole fractions are 0.12 and 0.35, respectively. If the partial pressure of the nitrogen is 0.8 atm, determine
(a) The total pressure in the tank,
(b) The total number of moles (kmol) in the tank,
(c) The mass of the mixture in the tank.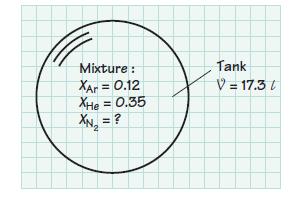
Transcribed Image Text:
Mixture : XAr = 0.12 XHe = 0.35 Tank V = 17.3 /
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 66% (6 reviews)
To solve this problem we will use the ideal gas law which states PV nRT where P is the pressure V is the volume n is the number of moles R is the gas ...View the full answer

Answered By

KELVIN MUCHIRI
I have experience teaching math, science, and English to students of all ages. I have also worked as a tutor in a college setting, helping students with their homework and preparing them for exams.
I believe that tutoring is a great way to help students learn. It allows students to get one-on-one help with their studies, and it gives them the chance to ask questions and get immediate feedback. Tutoring can also be tailored to the individual needs of the student, which is why I believe it is so effective.
I have seen firsthand how tutoring can help students improve their grades and confidence. I have also seen how it can help students who are struggling with a particular subject. I believe that tutoring is a great way to help students learn and succeed in school.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
1 723 Conditions for promotions 5 Years of service (Years) 6 Psychometric test (%) Required: a) LIST OF EMPLOYEES FOR PROMOTION 9 Names of employees Years of service 10 Munawarah Ali 11 Amiruddin...
-
The following additional information is available for the Dr. Ivan and Irene Incisor family from Chapters 1-5. Ivan's grandfather died and left a portfolio of municipal bonds. In 2012, they pay Ivan...
-
At 298 K the standard enthalpy of combustion of sucrose is -5797 k] mol-I and the standard Gibbs energy of the reaction is -6333 k] mol ". Estimate the additional non-expansion work that may be...
-
You work for a gas turbine design company and have a client who has a fairly loose specification for a gas turbine engine. You are required to design an aviation gas turbine to power the aircraft...
-
At the end of 2012, Tootsie Roll had a price- earnings ratio of 30.7, and Hershey had a price- earnings ratio of 25.3. These convert to capitalization rates of 3.25% for Tootsie Roll and 3.95% for...
-
Case Development began operations in December 2024. When property is sold on an installment basis, Case recognizes installment income for financial reporting purposes in the year of the sale. For tax...
-
Outline the steps involved in: (a) planning (b) executing (c) reporting (d) following up an internal audit.
-
Analysis of return on invested assets, comparison of two divisions, DuPont method. Global Data, Inc., has two divisions: Test Preparation and Language Arts. Results (in millions) for the past three...
-
Applied vs. Actual Manufacturing Overhead Davis Manufacturing Corporation applies manufacturing overhead on the basis of 150% of direct labor cost. An analysis of the related accounts and job order...
-
Computer Wizards Ltd. has determined that its Net Income for Tax Purposes, before any CCA deductions, was $80,000, for the taxation year ending December 31, 2020. As Computer Wizards Ltd. does not...
-
Determine the total apparent specific heat at constant pressure (c p,mix in kJ/kg K) for a fuelair reactant mixture containing 1 kmol CH 4 , 2.5 kmol O 2 , and 9.4 kmol N 2 at 500 K and 1 atm. Use...
-
In Table C.2, at what reference temperature and pressure is the entropy zero? TABLE C.2 Thermodynamic Properties of Air at 1 atm* h (kJ/kg) u (kJ/kg) s (kJ/kg-K) 325.42 268.14 3.4764 335.49 275.32...
-
In preparing the financial statements of R Ltd for the year ended 31.3.2017, you come across the following information. State with reasons, how would you deal with them in the financial statements:...
-
Use Adams' plan in Problems 8-10. Show that it violates the quota rule. State: A 1.646 Population: Number of seats: 250 B C 2,091 154 D 6,937 E 685 F 988
-
Modified quotas are given in Problems 7-14. Round your answers to two decimal places. a. Find the lower and upper quotas. b. Find the arithmetic mean of the lower and upper quotas. c. Find the...
-
The Adobe School District is hiring a vice principal and has interviewed four candidates: Andrew (A), Bono (B), Carol (C), and Davy (D). The hiring committee members have indicated their preferences:...
-
Chemistry is taught at five high schools in the Santa Rosa Unified School District. The district has just received a grant of 100 microscopes which are to be apportioned to the five high schools...
-
In Problems 15-18, use Hamilton's plan to apportion the new seats to the existing states. Then increase the number of seats by one and decide whether the Ala bama paradox occurs. Assume that the...
-
Explain the progression from decision support systems to analytics through business intelligence.
-
The Ranch 888 Noodle Company sells two types of dried noodles:ramen, at $6.50 per box, and chow fun, at $7.70 per box. So farthis year, the company has sold a total of 110,096 boxes ofnoodles,...
-
Using the results of exercises 9 and 10, determine the mass air-to-fuel ratio (A/F) mass for the combustion of natural gas in air. (A: 17.2 kg of air/kg of fuel)
-
Determine the mass air-to-fuel ratio (A/F) mass for the combustion of an oil (represented by CH 2 ) in air.
-
Determine the mass air-to-fuel ratio (A/F)mass for the combustion of coal in air represented by the equation: CHN 0:01 O 0:1 S 0:05 + a(O 2 + 3:76N 2 ) = bCO 2 + cH 2 O + dN 2 + eSO 2
-
Using the following information: a. The bank statement balance is $3,048. b. The cash account balance is $3,300. c. Outstanding checks amount to $755. d. Deposits in transit are $809. e. The bank...
-
Determine the average tax rate and the marginal tax rate for each of the following instances: Use the Tax Tables for taxpayers with taxable income under $ 1 0 0 , 0 0 0 and the Tax Rate Schedules for...
-
A machine was bought on 1 st January 2 0 1 7 for $ 1 2 , 0 0 0 . The policy is to depreciate the machine at 1 0 % on reducing balance method at the end of each year, ending on 3 1 st December....

Study smarter with the SolutionInn App


