A mixture of ideal gases contains 1 kmol of CO 2 , 2 kmol of H 2
Question:
A mixture of ideal gases contains 1 kmol of CO2, 2 kmol of H2O, 0.1 kmol of O2, and 7.896 kmol of N2. Determine the mole fractions and the mass fractions of each constituent. Also determine the apparent molecular weight of the mixture.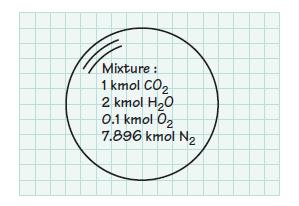
Transcribed Image Text:
Mixture: 1 kmol CO₂ 2 kmol H₂O 0.1 kmol O₂ 7.896 kmol N₂
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
To calculate the mole fractions we need to find the total number of moles in the mixture Total numbe...View the full answer

Answered By

KELVIN MUCHIRI
I have experience teaching math, science, and English to students of all ages. I have also worked as a tutor in a college setting, helping students with their homework and preparing them for exams.
I believe that tutoring is a great way to help students learn. It allows students to get one-on-one help with their studies, and it gives them the chance to ask questions and get immediate feedback. Tutoring can also be tailored to the individual needs of the student, which is why I believe it is so effective.
I have seen firsthand how tutoring can help students improve their grades and confidence. I have also seen how it can help students who are struggling with a particular subject. I believe that tutoring is a great way to help students learn and succeed in school.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley
Question Posted:
Students also viewed these Engineering questions
-
A mixture of ideal gases contains 0.5 kmol of CO 2 , 2 kmol of O 2 , and 7 kmol of N 2 at 700 K. Determine the following quantities: A. The mole fraction of each constituent in the mixture. B. The...
-
A mixture of ideal gases is made up of 30 percent N2, 30 percent O2, and 40 percent H2O by mole fraction. Determine the Gibbs function of the N2 when the mixture pressure is 5 atm, and its...
-
A mixture of ideal gases flowing at 50 kg/min is compressed from 100 kPa and 20 8 C to 600 kPa in an insulated compressor. Determine the minimum horsepower requirement if the gravimetric analysis of...
-
Which of the following is not necessary to do before you can run a Java program? a. Coding b. Compiling c. Debugging d. Saving
-
Define business valuation and discuss the challenge of valuing private or closely held companies.
-
A project has the following cash flows : year cash flows 0 -10,700 1 4,510 2 6,460 3 4,000 4 -1,840 Assuming the appropriate interest rate is 9...
-
Indicate the audit procedure(s) you would use to verify each of the following items. Briefly explain each procedure, indicating how it would be applied in the particular situation. (i) Value of...
-
A Gallup Daily Tracking Survey found that the mean daily discretionary spending by Americans earning over $90,000 per year was $136 per day (USA today, July 30, 2012). The discretionary spending...
-
Data for Hermann Corporation are shown below: Selling price Variable expenses Contribution margin Per Unit Percent of Sales $ 110 77 100% 70 $ 33 30% Fixed expenses are $82,000 per month and the...
-
Enter the missing dollar amounts for the income statement for each of the following independent cases: Cases Sal les Beginning Purchases Total Revenue Inventory EndingCostGross Expenses Pretax...
-
Determine the apparent molecular weight of synthetic air created by mixing 1 kmol of O 2 with 3.76 kmol of N 2 . Also determine the mole and mass fractions of the O 2 in the mixture. 0- N D Synthetic...
-
For air containing 75.53% N 2 , 23.14% O 2 , 1.28% Ar, and 0.05% CO 2 , by mass, determine the gas constant and its molecular weight. How do these values compare for a mass based composition of 76.7%...
-
The following data were extracted from the income statement of Shriver Inc.: a. Determine for each year (1) The inventory turnover and (2) The number of days sales in inventory. Round to the nearest...
-
Compute the limit of the convergent sequences in Problems 19-26. \(\left\{\frac{8 n^{2}+800 n+5,000}{2 n^{2}-1,000 n+2}ight\}\)
-
Find each limit in Problems 11-18, if it exists. \(\lim _{n ightarrow \infty} \frac{2 n+1}{3 n-4}\)
-
Find each limit in Problems 11-18, if it exists. \(\lim _{n ightarrow \infty} \frac{4 n-1}{3 n+10}\)
-
Find the antiderivative by using areas in Problems 9-22. \(\int 5 d x \)
-
What is the Pareto principle?
-
List five claimed limitations of independent data marts.
-
Complete the equations for the following equilibria and calculate Keq where the Keq expression includes [HO]. Be sure to enter Keq in proper scientific notation. (a) ammonia (acting as a base) reacts...
-
A vehicle has a 15. US gallon gas tank and can be filled from empty in 60. seconds. a. What is the rate that power is transferred to the vehicle? b. If the vehicle is converted to an all-battery...
-
A windmill produces mechanical power according to this formula: P = 1/2rV 3 A where Z is its efficiency (assume Z = 60.%), r = density of air (1.00 kg/m 3 ), V = wind speed in m/s (assume 5.0 m/s),...
-
You wish to store 2.00 MWh as an emergency power supply for a big-box store. If the gross energy storage density of the battery is 425 kJ/liter how big is the storage battery in m 3 ? If the density...
-
Honolua Surf Company has two classes of capital stock outstanding: 8%, $20 par preferred and $5 par common. On December 31, 2025, the following accounts were reported in Stockholders' Equity:...
-
A) B) Jenny Corporation recorded warranty accruals as at December 31, 2019, in the amount of $150,000. This reversing difference will cause deductible amounts of $50,000 in 2020, $35,000 in 2021, and...
-
es Fetzer Corporation provided the following partial-trial balance for the current year. (Click the icon to view the partial-trial balance.) Prepare a statement of net income and additional...

Study smarter with the SolutionInn App


