Consider air (ideal gas) within a pistoncylinder assembly as a thermodynamic system. We wish to compare three
Question:
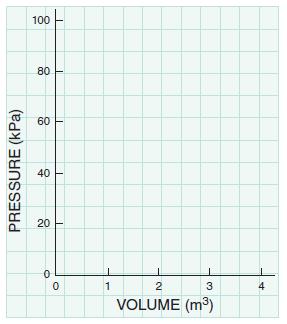
Consider air (ideal gas) within a piston–cylinder assembly as a thermodynamic system. We wish to compare three different quasi-equilibrium expansion processes for this system by plotting the pressure as a function of volume. Each process starts from the same initial condition: P = 100 kPa and V = 1 m3. The final volume for each process is V = 3 m3. The plots are to be made by connecting three points (V = 1, 2, and 3m3) with hand-drawn lines. The first process (trivial) is conducted at constant pressure. The second process is conducted at a constant temperature. The third process is conducted such that PV1.4 is maintained constant.
A. Complete the table below by calculating the six values for the pressure.
B. Plot the results on the graph axes given here or on graph paper. Connect the data points with a smooth line.
C. Using your plots, rank the quantity of work done by the three processes from the least to the most.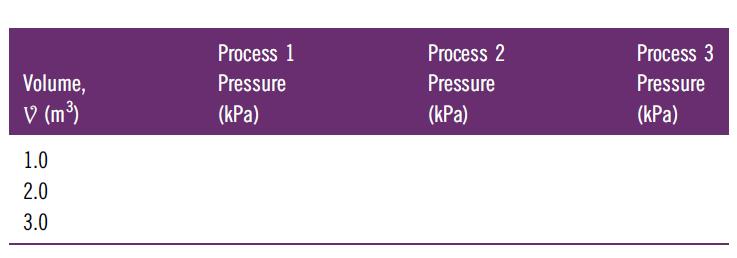
Step by Step Answer:

Thermodynamics Concepts And Applications
ISBN: 9781107179714
2nd Edition
Authors: Stephen R. Turns, Laura L. Pauley





