3 kg of ammonia at its critical state is placed in a constant-volume container, which is then...
Question:
3 kg of ammonia at its critical state is placed in a constant-volume container, which is then cooled until the temperature is 300 K. How a much energy was transferred as heat from the ammonia in this process, and what tank volume is occupied by liquid at the end of the process? Use the tables in Appendix A.7 for data.
Data From Appendix A.7
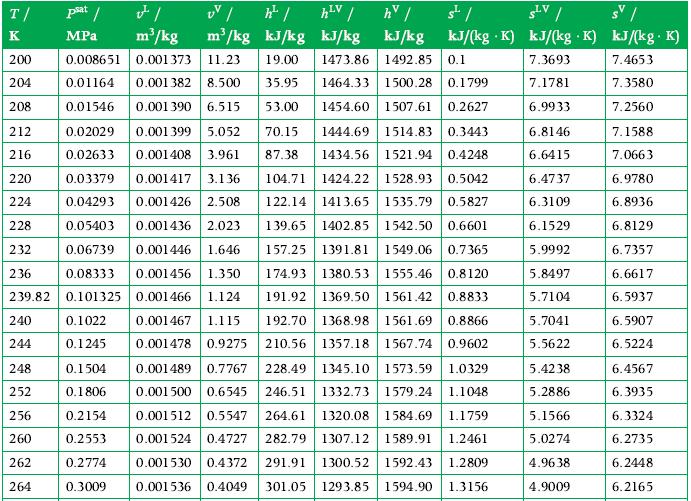
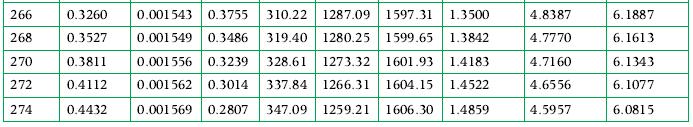
Transcribed Image Text:
T/ K UV / ¹² / of / m³/kg MPa m³/kg kJ/kg 200 0.008651 0.001373 11.23 19.00 204 0.01164 0.001 382 8.500 35.95 208 0.01546 0.001 390 6.515 53.00 212 0.02029 0.001 399 5.052 70.15 1444.69 1514.83 0.3443 216 0.02633 0.001408 3.961 87.38 1434.56 1521.94 0.4248 220 224 228 232 0.03379 0.001417 3.136 104.71 1424.22 1528.93 0.5042 0.04293 0.001426 2.508 122.14 1413.65 1535.79 0.5827 0.05403 0.001436 2.023 139.65 1402.85 1542.50 0.6601 0.06739 0.001446 1.646 157.25 1391.81 1549.06 0.7365 236 0.08333 0.001456 1.350 174.93 1380.53 1555.46 0.8120 239.82 0.101325 0.001466 1.124 191.92 1369.50 1561.42 0.8833 240 0.1022 0.001467 1.115 192.70 1368.98 1561.69 0.8866 0.1245 210.56 1357.18 1567.74 0.9602 244 0.001478 0.9275 0.001489 0.7767 228.49 1345.10 1573.59 1.0329 0.001500 0.6545 246.51 1332.73 1579.24 1.1048 0.001512 0.5547 264.61 1320.08 1584.69 1.1759 0.001524 0.4727 282.79 1307.12 1589.91 1.2461 0.001530 0.4372 291.91 1300.52 1592.43 1.2809 0.001536 0.4049 301.05 1293.85 1594.90 1.3156 248 252 256 260 262 264 0.1504 0.1806 hv / "/ sv/ kJ/kg kJ/(kg K) kJ/(kg K) kJ/(kg. K) 7.3693 7.4653 7.1781 7.3580 6.9933 7.2560 6.8146 7.1588 6.6415 7.0663 6.4737 6.3109 6.1529 5.9992 5.8497 5.7104 5.7041 5.5622 0.2154 0.2553 0.2774 0.3009 hLV | kJ/kg 1473.86 1492.85 0.1 1464.33 1500.28 0.1799 1454.60 1507.61 0.2627 SLV 5.4238 5.2886 5.1566 5.0274 4.9638 4.9009 6.9780 6.8936 6.8129 6.7357 6.6617 6.5937 6.5907 6.5224 6.4567 6.3935 6.3324 6.2735 6.2448 6.2165
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 50% (2 reviews)
The energy balance for the iso...View the full answer

Answered By

Swarnim Raut
I tutored few juniors of mine during my Mechanical Engineering and my high school. I have the ability to explain difficult concepts into simple manner. My love for Mechanical Engineering has allowed me to get in depth knowledge of the subject. I like to learn and share it with others.
0.00
0 Reviews
10+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
Please need HELP ASAP! FOLLOW THE INSTRUCTIONS!! Problem 3: Presented below is information related to Macaw Corp., for the year 2019. Net sales Cost of goods sold Selling expenses Administrative...
-
Propane is in a 5 m 3 container as a saturated vapor at 300 K. The sealed tank is then cooled to 260 K by fresh snow. What are the pressure (Pa) and liquid volume in this 260 K state, and how much...
-
One kilogram of R134a is heated in a constant volume container from the critical point to 450 K. How much energy is transferred as heat to the fluid? Use the diagrams or data in Appendix A.4. Data...
-
Design a plan to research and select a new or used automobile.
-
The pre-closing, year-end trial balance for a capital projects fund of the city of Rochester as of December 31, 2019, follows: Required 1. Prepare closing entries as of December 31, 2019. 2. Prepare...
-
Ellen, Fargo, and Gary are partners who share profits and losses 20 percent, 20 percent, and 60 percent, respectively, after Ellen and Fargo each receive a $12,000 salary allowance. Capital balances...
-
In the ammonia synthesis reaction \(\mathrm{N}_{2}+3 \mathrm{H}_{2}=2 \mathrm{NH}_{3}+22.4 \mathrm{kcal}\), the formation of \(\mathrm{NH}_{3}\) will be favoured by (a) High temperature (b) Low...
-
The standard cost card for the single product manufactured by Cutter, Inc., is given below: Manufacturing overhead is applied to production on the basis of standard direct labor-hours. During the...
-
Given the below Language L (where = {a, b}), use the Pumping Lemma to show that L is not regular. (Solve) l L= {ba" ab" | n > 0}
-
The planet and its moon gravitationally attract each other. Rank the forces of attraction between each pair, from greatest to least. B 2M (2m (2m d 2d
-
A free-piston engine under development consists of a small piston in a cylinder. Each end of the cylinder behaves like a two-stroke engine, in which the following sequence of processes occurs...
-
Determine the equivalents of 1 m, 1 kg, 1 N, 1 J, and 1 W in a Systme Unis in which both k N and k G are unity, the speed of light is unity, and the second is the only primary unit. Choose a suitable...
-
At December 31, 2024, Blanda Creations had a credit balance of 15,000 in Allowance for Doubtful Accounts. During 2025, Blanda wrote off accounts totaling 11,000. One of those accounts (1,800) was...
-
Why does an investment have an opportunity cost rate even when the funds employed have no explicit cost?
-
A government entity that regulates an authorized monopoly will most likely base regulated prices on: A. marginal cost. B. long-run average cost. C. first-degree price discrimination.
-
How does the effective annual rate differ from the stated rate?
-
How does the periodic rate differ from the stated rate?
-
If companies earn economic profits in a perfectly competitive market, over the long run the supply curve will most likely: A. shift to the left. B. shift to the right. C. remain unchanged.
-
Compute C8,8.
-
What is your opinion of advertising awards, such as the Cannes Lions, that are based solely on creativity? If you were a marketer looking for an agency, would you take these creative awards into...
-
Michael Phelps won a record-setting 8 gold medals at the 2008 Beijing Olympics. Now imagine if Phelps had competed in a pool filled with pancake syrup. Would you expect his race times to increase,...
-
The fuel tank on a sport-utility wagon holds 14 gal of gasoline. How much heavier is the automobile when the tank is full compared to when it is empty?
-
The pressure at the bottom of an 18-ft-deep storage tank for gasoline is how much greater than at the top? Express your answer in the units of psi.
-
Dickinson Company has $12,080,000 million in assets. Currently half of these assets are financed with long-term debt at 10.4 percent and half with common stock having a par value of $8. Ms. Park,...
-
IFRS and U.S. GAAP are relatively similar with respect to current liabilities and contingencies. Relatively minor differences relate to when financing must be in place for a liability expected to be...
-
Required Information [The following Information applies to the questions displayed below.] Simon Company's year-end balance sheets follow. At December 31 Assets Current Year 1 Year Ago 2 Years Ago...

Study smarter with the SolutionInn App


