Compute the standardized enthalpy and entropy of a mixture formed by an equal mass amount of CO
Question:
Compute the standardized enthalpy and entropy of a mixture formed by an equal mass amount of CO2?and H2O at 2 atm and 311 K. If liquid is present for these conditions, assume that the vapor phase can be approximated as an ideal gas mixture, and the liquid phase is pure water. Use:?
a) Values obtained from Table A.1, and?
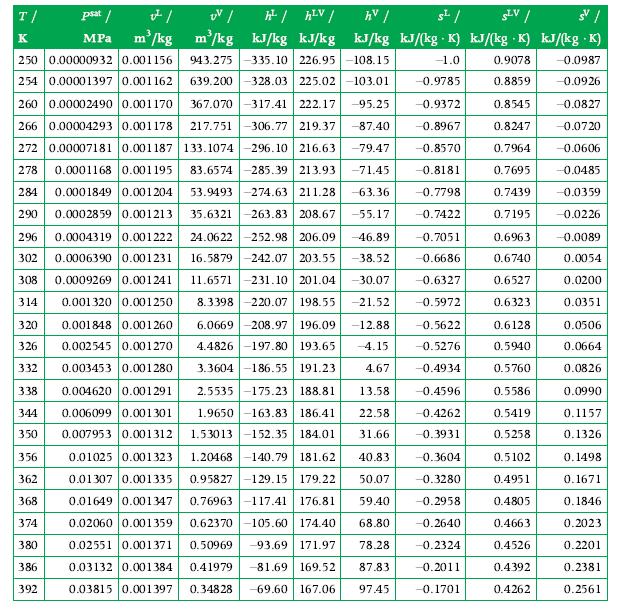
b) values obtained with GASMIX. If there is a difference, explain it.
Transcribed Image Text:
T/ psat / v²/ UV / h²/ hLV/ hv/ s²/ SLV/ SV/ K MPa m³/kg . m³/kg kJ/kg kJ/kg kJ/kg kJ/(kg K) kJ/(kg K) kJ/(kg .K) 943.275 -335.10 226.95-108.15 -1.0 250 0.00000932 0.001156 254 0.00001397 0.001162 260 0.00002490 0.001170 367.070-317.41 222.17 -95.25 639.200-328.03 225.02-103.01 -0.9785 -0.9372 -87.40 -0.8967 -79.47 -0.8570 -71.45 -0.8181 -63.36 -0.7798 -0.7422 266 0.00004293 0.001178 217.751-306.77 219.37 272 0.00007181 0.001187 133.1074 296.10 216.63 278 0.0001168 0.001195 83.6574 -285.39 213.93 284 0.0001849 0.001204 53.9493 -274.63 211.28 290 0.0002859 0.001213 35.6321-263.83 208.67 -55.17 296 0.0004319 0.001222 24.0622 -252.98 206.09 -46.89 302 0.0006390 0.001231 16.5879 -242.07 203.55 -38.52 308 0.0009269 0.001241 11.6571-231.10 201.04 -30.07 0.001320 0.001250 8.3398 220.07 198.55 -21.52 0.001848 0.001260 6.0669 -208.97 196.09 -12.88 326 0.002545 0.001270 4.4826 -197.80 193.65 -4.15 332 0.003453 0.001280 3.3604-186.55 191.23 338 0.004620 0.001291 314 320 4.67 2.5535-175.23 188.81 13.58 -0.4596 -0.4262 1.9650 -163.83 186.41 22.58 1.53013-152.35 184.01 31.66 -0.3931 344 0.006099 0.001301 350 0.007953 0.001312 356 0.01025 0.001323 0.01307 0.001335 0.01649 0.001347 -0.3604 362 -0.3280 1.20468-140.79 181.62 40.83 0.95827-129.15 179.22 50.07 0.76963-117.41 176.81 59.40 0.62370-105.60 174.40 68.80 368 -0.2958 374 0.02060 0.001359 -0.2640 380 0.50969 -93.69 171.97 78.28 -0.2324 0.02551 0.001371 0.03132 0.001384 0.41979 -81.69 169.52 87.83 386 -0.2011 392 0.03815 0.001397 0.34828 -69.60 167.06 97.45 -0.1701 -0.7051 -0.6686 -0.6327 -0.5972 -0.5622 -0.5276 -0.4934 0.9078 -0.0987 0.8859 -0.0926 0.8545 -0.0827 0.8247 -0.0720 0.7964 -0.0606 0.7695 -0.0485 0.7439 -0.0359 0.7195 -0.0226 0.6963 0.6740 0.6527 0.6323 0.6128 0.5940 0.5760 0.5586 0.5419 0.5258 0.5102 0.4951 0.4805 0.4663 0.4526 0.4392 0.4262 -0.0089 0.0054 0.0200 0.0351 0.0506 0.0664 0.0826 0.0990 0.1157 0.1326 0.1498 0.1671 0.1846 0.2023 0.2201 0.2381 0.2561
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Answer rating: 60% (10 reviews)
a b From Appendix A1 we have MHo 001802 kgkmol and Mco 004401 kgkmol ...View the full answer

Answered By

Cristine kanyaa
I possess exceptional research and essay writing skills. I have successfully completed over 5000 projects and the responses are positively overwhelming . I have experience in handling Coursework, Session Long Papers, Manuscripts, Term papers, & Presentations among others. I have access to both physical and online library. this makes me a suitable candidate to tutor clients as I have adequate materials to carry out intensive research.
4.90+
1538+ Reviews
3254+ Question Solved
Related Book For 

Thermodynamics Fundamentals And Engineering Applications
ISBN: 9780521862738
1st Edition
Authors: William C. Reynolds, Piero Colonna
Question Posted:
Students also viewed these Engineering questions
-
A thin (1.0-mm-thick) coat of fresh paint has just been sprayed over a 1.5-m by 1.5-m square steel body part, which approximates a flat surface. The paint contains a volatile solvent that initially...
-
What is meant by Cumulative Record Card?
-
Briefly explain the Nature and purpose of the Cumulative Record Card (CRC) along with the design and advantages of the CRC.
-
Find the radius of convergence and interval of convergence of the series. 00 (-1)"x" n=0 n + 1
-
Nordau and Boyer (2000) estimated that the United States would bear over 90% of the total world cost of achieving the Kyoto targets for greenhouse gas emission reductions. Explain how this can be,...
-
Debra and Merina sell electronic equipment and supplies through their partnership. They wish to expand their computer lines and decide to admit Wayne to the partnership. They share income in a ratio...
-
The feed to a pentane isomerization process consists of \(650 \mathrm{kmol} / \mathrm{hr}\) of n-pentane and \(300 \mathrm{kmol} / \mathrm{hr}\) of isopentane. The effluent from the catalytic...
-
Dolphin Ceramics produces large planters to be used in urban landscaping projects. A special earth clay is used to make the planters. The standard quantity of clay used for each planter is 24 pounds....
-
Explain the environmental and safety considerations in crystallization processes, focusing on strategies to minimize hazardous waste, reduce energy consumption, and ensure process safety through...
-
Ashton and Melody Webb are a married couple in their mid-20s. Ashton has a good start as an electrical engineer and Melody works as a sales representative. Since their marriage four years ago, Ashton...
-
Obtain the expression for the specific flow exergy (9.5) from its definition.
-
Derive the reference I-law cycle efficiency for the evaluation of the II-law efficiency of refrigeration systems.
-
Without looking up any data, make an order-of-magnitude estimate of the annual consumption of gasoline (in gallons) by passenger cars in the United States. Make reasonable order-of-magnitude...
-
Based on the following information, what is the year 1 income statement, Balance sheet end of year 1, Year 1 cash flow, and must sell in order to sell to break even. ?I have uploaded the template to...
-
Jefferson Products, Inc., is considering purchasing a new automatic press brake, which costs $320,000 including installation and shipping. The machine is expected to generate net cash inflows of...
-
Yuppie town has two food stores, LA Boulangerie, which sells bread, and La Fromagerie, which sells cheese. It costs $1 to make a loaf of bread and $2 to make a pound of cheese. If La Boulangerie's...
-
Discuss howallocations are undertaken between land and composite commodities in a monocentric city.
-
es Walton Airlines is a small airline that occasionally carries overload shipments for the overnight delivery company Never-Fail, Incorporated. Never-Fail is a multimillion-dollar company started by...
-
Calculate the income yield, capital gain yield, and rate of total return in each of 2008 and 2009 for Potash Corporations shares and Mawer New Canada Fund units. Use the data in Tables 9.3 and 9.4.
-
Open Text Corporation provides a suite of business information software products. Exhibit 10-9 contains Note 10 from the companys 2013 annual report detailing long-term debt. Required: a. Open Text...
-
Explain why the solubility of an ionic compound increases as the ionic strength of the solution increases (at least up to ~ 0.5 M).
-
Which statements are true? In the ionic strength range 00.1 M, activity coefficients decrease with (a) Increasing ionic strength; (b) Increasing ionic charge; (c) Decreasing hydrated radius.
-
Calculate the activity coefficient of Al 3+ when = 0.083 M by linear interpolation in Table 7-1. Table 7-1 Ion size Ionic strength (p. M) Ion (a, pm) 0.001 0.005 0.01 0.05 0.1 Charge = +1 H*...
-
Many companies have been experimenting with variations of a "Pay What You Want" pricing schemes. One of the earliest attempts at this type of schemes is Radiohead's 2007 In Rainbows album (fans could...
-
What is a recommendation you could make about brand positioning and/or branding strategy to help build the brand and contribute to align it with what your target segment wants. How will this...
-
In a marketing plan pricing and positioning strategy must be aligned. For example, the want for the company is to be known as the premier brand, having too low a price might dissuade customers from...

Study smarter with the SolutionInn App


