In exercise 3.6.2 devoted to the thermalisation of two blocks, show for the particular case where N1
Question:
In exercise 3.6.2 devoted to the thermalisation of two blocks, show for the particular case where N1 = N2 = N that the entropy variation,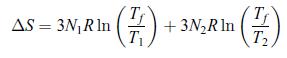 is strictly positive.
is strictly positive.
Data from exercise 3.6.2
The entropy of a particular substance is given in terms of its internal energy U and number of moles N as [31],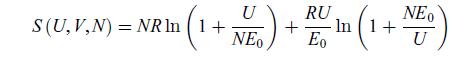
where R and E0 are positive constants. A system consists of two subsystems containing such a substance, with NA moles in subsystem A and NB moles of it in subsystem B. When the subsystems are set in thermal contact, their initial temperatures are TiA
and TiB. Determine the final temperature Tf of the system.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Principles Of Thermodynamics
ISBN: 9781108426091
1st Edition
Authors: Jean-Philippe Ansermet, Sylvain D. Brechet
Question Posted:





