Question: You are conducting experiments to study prototype heat engines. In one test, 4.00 mol of argon gas are taken around the cycle shown in Fig.
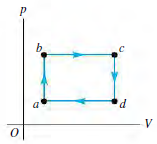
You are conducting experiments to study prototype heat engines. In one test, 4.00 mol of argon gas are taken around the cycle shown in Fig. P20.57. The pressure is low enough for the gas to be treated as ideal. You measure the gas temperature in states a, b, c, and d and find Ta= 250.0 K, Tb= 300.0 K, Tc= 380.0 K, and Td= 316.7 K.
(a) Calculate the efficiency e of the cycle.
(b) Disappointed by the cycle€™s low efficiency, you consider doubling the number of moles of gas while keeping the pressure and volume the same. What would e be then?
(c) You remember that the efficiency of a Carnot cycle increases if the temperature of the hot reservoir is increased. So, you return to using 4.00 mol of gas but double the volume in states c and d while keeping the pressures the same. The resulting temperatures in these states are Tc = 760.0 K and Td = 633.4 K. Ta and Tb remain the same as in part (a). Calculate e for this cycle with the new Tc and Td values.
(d) Encouraged by the increase in efficiency, you raise Tc and Td still further. But e doesn€™t increase very much; it seems to be approaching a limiting value. If Ta = 250.0 K and Tb = 300.0 K and you keep volumes Va and Vb the same as in part (a), then Tc/Td = Tb/Ta and Tc = 1.20Td. Derive an expression for e as a function of Td for this cycle. What value does e approach as Td becomes very large?
Figure P20.57
b.
Step by Step Solution
3.55 Rating (165 Votes )
There are 3 Steps involved in it
IDENTIFY and SET UP The cycle consists of two isochoric processes ab and cd and two isobaric process... View full answer

Get step-by-step solutions from verified subject matter experts


