Question: The feed to a continuous crystallizer that can be simulated with the MSMPR model is 5,000 kg/h of 40 wt% sodium acetate in water. Monoclinic
The feed to a continuous crystallizer that can be simulated with the MSMPR model is 5,000 kg/h of 40 wt% sodium acetate in water. Monoclinic crystals of the trihydrate will be formed. The pressure in the crystallizer and the heat-transfer rate in the associated heat exchanger are such that 20% of the water in the feed will be evaporated at a crystallizer temperature of 40°C. The crystal growth rate, G, is 0.0002 m/h and a predominant crystal size, Lpd, of 20 mesh is desired.
Determine:
(a) The kg/h of crystals in the exiting magma.
(b) The kg/h of mother liquor in the exiting magma.
(c) The volume in m3 of magma in the crystallizer if density of the crystals = 1.45 g/cm3 density of the mother liquor = 1.20 g/cm3
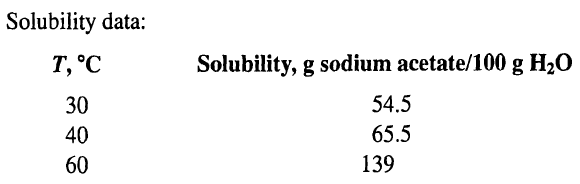
Solubility data: Solubility, g sodium acetate/100 g H2O T, C 54.5 65.5 139 30 40 60
Step by Step Solution
3.52 Rating (166 Votes )
There are 3 Steps involved in it
a and b Compute a mass balance around the crystallizer The feed contains 045000 2000 k... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (589).docx
120 KBs Word File


