Question: Example 17-14, part B, illustrates a method to increase the size of product crystals by seeding. An alternative procedure that might be feasible in some
Example 17-14, part B, illustrates a method to increase the size of product crystals by seeding. An alternative procedure that might be feasible in some situations is to keep supersaturation, \(\mathrm{V}, \mathrm{Q}_{\text {out }}\), and \(\Delta \mathrm{L}_{\text {growth }}\) constant and increase the size of the seeds, \(\mathrm{L}_{\mathrm{S}}\), to obtain desired product size, \(\mathrm{L}_{\mathrm{p}}\). Repeat Example 17-14, part b, to obtain a particle size of \(\mathrm{L}_{\mathrm{p}}\) \(=0.60105 \mathrm{~mm}\) with constant supersaturation, \(\tau\), and \(\Delta \mathrm{L}_{\text {growth }}\) but with larger seeds. Find new values of \(\mathrm{L}_{\mathrm{S}}\) and of \(\mathrm{W}_{\mathrm{S}}\).
Example 17-14
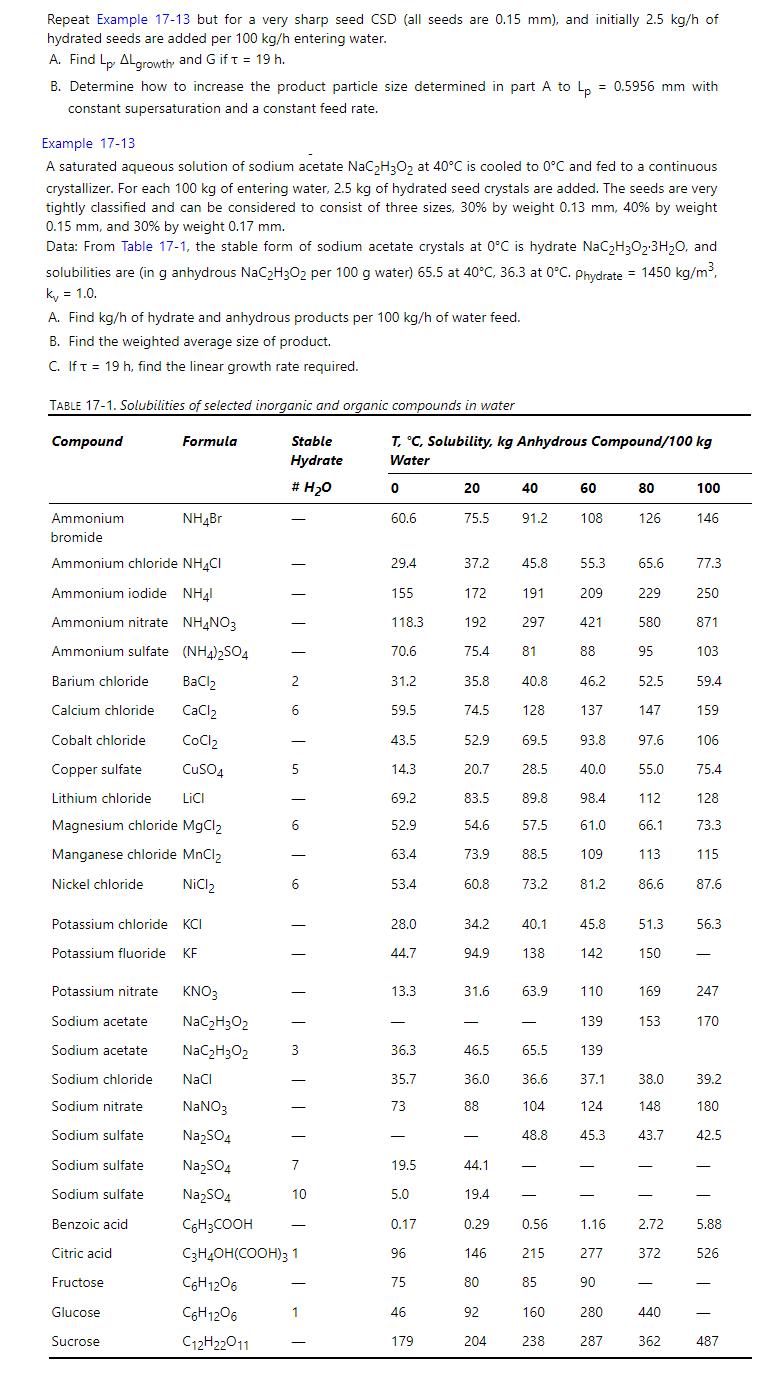
Repeat Example 17-13 but for a very sharp seed CSD (all seeds are 0.15 mm), and initially 2.5 kg/h of hydrated seeds are added per 100 kg/h entering water. A. Find Lp. ALgrowth and G if t = 19 h. B. Determine how to increase the product particle size determined in part A to Lp constant supersaturation and a constant feed rate. Example 17-13 0.5956 mm with A saturated aqueous solution of sodium acetate NaC2H3O2 at 40C is cooled to 0C and fed to a continuous crystallizer. For each 100 kg of entering water, 2.5 kg of hydrated seed crystals are added. The seeds are very tightly classified and can be considered to consist of three sizes, 30% by weight 0.13 mm, 40% by weight 0.15 mm, and 30% by weight 0.17 mm. Data: From Table 17-1, the stable form of sodium acetate crystals at 0C is hydrate NaC2H3O2.3HO, and solubilities are (in g anhydrous NaC2H3O2 per 100 g water) 65.5 at 40C, 36.3 at 0C. Phydrate = 1450 kg/m, kv = 1.0. A. Find kg/h of hydrate and anhydrous products per 100 kg/h of water feed. B. Find the weighted average size of product. C. If t = 19 h, find the linear growth rate required. TABLE 17-1. Solubilities of selected inorganic and organic compounds in water Compound Ammonium bromide Formula Stable Hydrate T, C, Solubility, kg Anhydrous Compound/100 kg Water # HO 0 20 40 60 80 100 NH4Br 60.6 75.5 91.2 108 126 146 Ammonium chloride NH4Cl 29.4 37.2 45.8 55.3. 65.6 77.3 Ammonium iodide NH4 155 172 191 209 229 250 Ammonium nitrate NH4NO3 118.3 192 297 421 580 871 Ammonium sulfate (NH4)2SO4 70.6 75.4 81 88 95 103 Barium chloride BaCl2 2 31.2 35.8 40.8 46.2 52.5 59.4 Calcium chloride CaCl2 6 59.5 74.5 128 137 147 159 Cobalt chloride CoCl2 43.5 52.9 69.5 93.8 97.6 106 Copper sulfate CuSO4 5 14.3 20.7 28.5 40.0 55.0 75.4 Lithium chloride LiCI 69.2 83.5 89.8 98.4 112 128 Magnesium chloride MgCl2 6 52.9 54.6 57.5 61.0 66.1 73.3 Manganese chloride MnCl2 63.4 73.9 88.5 109 113 115 Nickel chloride NiCl2 6 53.4 60.8 73.2 81.2 86.6 87.6 Potassium chloride KCI 28.0 34.2 40.1 45.8 51.3 56.3 Potassium fluoride KF 44.7 94.9 138 142 150 Potassium nitrate KNO3 13.3 31.6 63.9 110 169 247 Sodium acetate NaC2H3O2 - 139 153 170 Sodium acetate NaCHO 3 36.3 46.5 65.5 139 Sodium chloride NaCl 35.7 36.0 36.6 37.1 38.0 Sodium nitrate NaNO3 73 88 104 124 148 Sodium sulfate Na2SO4 - 48.8 45.3 43.7 Sodium sulfate Na2SO4 7 19.5 44.1 Sodium sulfate Na504 10 5.0 19.4 Benzoic acid C6H3COOH 0.17 0.29 0.56 Citric acid C3H4OH(COOH)3 1 96 146 215 Fructose C6H12O6 75 80 85 Glucose C6H12O6 1 46 92 160 | h 8 8 | | 1.16 2.72 277 372 90 - 440 Sucrose C12H22O11 179 204 238 287 362 | | | 39.2 180 42.5 5.88 526 487
Step by Step Solution
3.33 Rating (159 Votes )
There are 3 Steps involved in it

Get step-by-step solutions from verified subject matter experts


