Question: The liquid-liquid extractor in figure operates at 100F and a nominal pressure of 15 psia. For the feed and solvent flows shown, determine the number
The liquid-liquid extractor in figure operates at 100°F and a nominal pressure of 15 psia. For the feed and solvent flows shown, determine the number of equilibrium stages to extract 99.5% of the acetic acid, using the NRTL equation for activity coefficients. The NRTL constants may be taken as follows:
1 = ethyl acetate
2 = water
3 = acetic acid
Compare the computed compositions of the raffinate and extract products to those of figure.
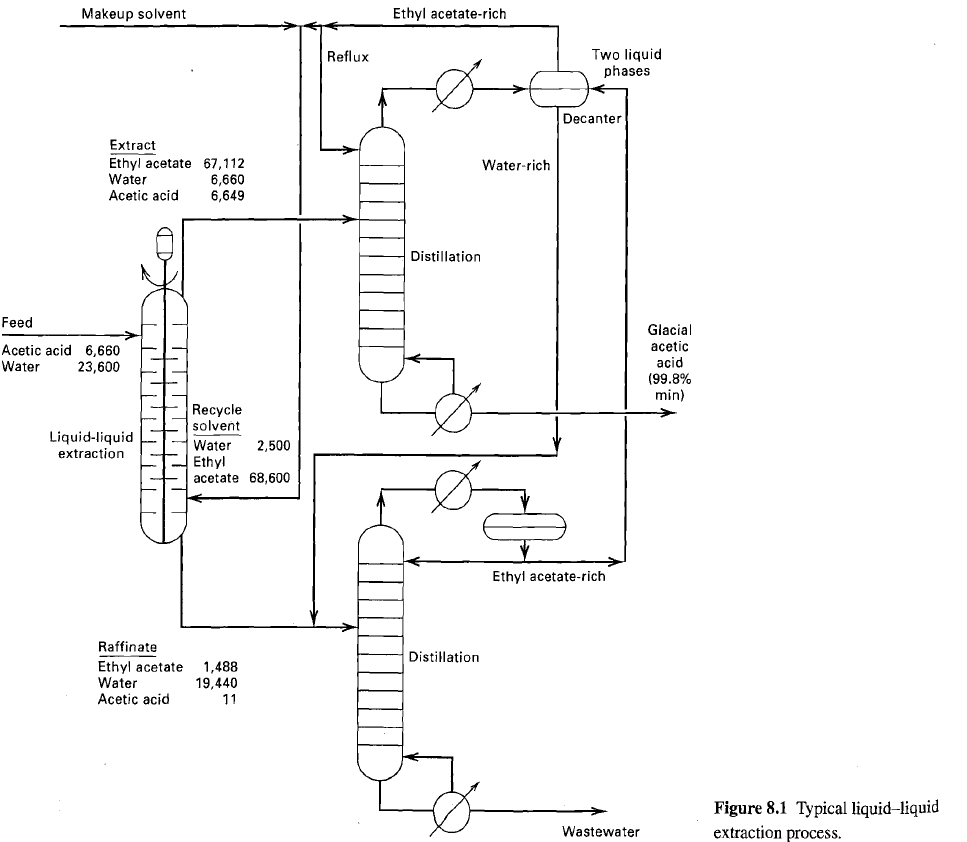
Ethyl acetate-rich Makeup solvent Two liquid phases Reflux Decanter Extract Ethyl acetate 67,112 Water Acetic acid Water-rich 6,660 6,649 Distillation Feed Glacial acetic acid Acetic acid 6,660 23,600 Water (99.8% min) Recycle solvent Liquid-liquid extraction Water Ethyl acetate 68,600 2,500 Ethyl acetate-rich Raffinate Distillation Ethyl acetate Water Acetic acid 1,488 19,440 11 Figure 8.1 Typical liquidliquid extraction process. Wastewater
Step by Step Solution
3.46 Rating (166 Votes )
There are 3 Steps involved in it
The Extract model of Chemcad was used starting with 2 equilibrium stages an... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
37-E-C-E-S-P (406).docx
120 KBs Word File


