The pH of water has great significance to environmental and chemical engineers. It can be related to
Question:
The pH of water has great significance to environmental and chemical engineers. It can be related to processes ranging from pipe corrosion to acid rain. The pH is related to the hydrogen ion concentration by
pH = ? log 10[H+]
The following five equations govern the concentrations of a mixture of carbon dioxide and water for a closed system:
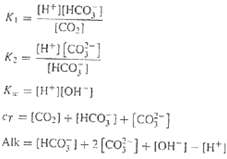
Where Alk = the alkalinity, cT = total inorganic carbon, and the K?s are equilibrium coefficients. The five unknowns are [CO2] = carbon dioxide, [HCO3?] = bicarbonate, [CO23?] = carbonate, [H+] = hydrogen ion, and [OH?] = hydroxyl ion. Solve for the five unknowns given that Alk = 2 x 10?3, cT = 3 x 10?3, Kl = 10?6.3, K2 = 10?10.3, and K w = 10?14. Also, calculate the solution?s pH.
Step by Step Answer:

Numerical Methods For Engineers
ISBN: 9780071244299
5th Edition
Authors: Steven C. Chapra, Raymond P. Canale





