Question: This problem can be worked by calculator or with the spreadsheet in Figure 18-5. Consider compounds X and Y in the example labeled Analysis of
This problem can be worked by calculator or with the spreadsheet in Figure 18-5. Consider compounds X and Y in the example labeled "Analysis of a Mixture, Using Equations 18-6" in Section 18-1. Find [X] and [Y] in a solution whose absorbance is 0.233 at 272 nm and 0.200 at 327 nm in a 0.100-cm cell.
Figure 18-5
![DE 1 Solving Simultaneous Linear Equations with Excel Matrix Operations A в F G Wavelength Coefficient Matrix Absorbance Concentrations 4. of unknown in mixture 5 16400 4.4304E-05 [X] -[Y] 272 3870 0.957 327 3990 6420 0.559 5.9537E-05 7 K A 9 1. Enter matrix of coefficients eb in cells B5:C6](https://dsd5zvtm8ll6.cloudfront.net/si.question.images/images/question_images/1591/8/0/1/2685ee0f5b4c96261591801261927.jpg)
Equation 18-6
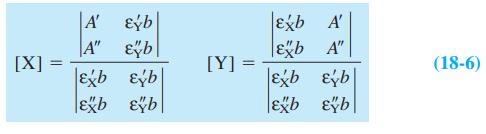
DE 1 Solving Simultaneous Linear Equations with Excel Matrix Operations A F G Wavelength Coefficient Matrix Absorbance Concentrations 4. of unknown in mixture 5 16400 4.4304E-05 [X] -[Y] 272 3870 0.957 327 3990 6420 0.559 5.9537E-05 7 K A 9 1. Enter matrix of coefficients eb in cells B5:C6 10 2. Enter absorbance of unknown at each wavelength (cells D5:D6) 11 3. Highlight block of blank cells required for solution (F5 and F6) 12 4. Type the formula "= MMULT(MINVERSE(B5:C6),D5:D6)" 5. Press CONTROL+SHIFT+ENTER on a PC or COMMAND+RETURN on a Mac 6. Behold! The answer appears in cells F5 and F6 13 14
Step by Step Solution
3.46 Rating (179 Votes )
There are 3 Steps involved in it
Putting b 0100 cm into the determinants giv... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2163).docx
120 KBs Word File


