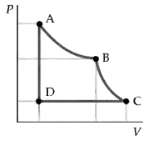
Two moles of a diatomic gas are carried through the cycle ABCDA shown in the PV diagram
Question:
Two moles of a diatomic gas are carried through the cycle ABCDA shown in the PV diagram in figure. The segment AB represents an isothermal expansion, the segment BC an adiabatic expansion. The pressure and temperature at A are 5 atm and 600 K. The volume at B is twice that at A. The pressure at D is 1atm.
(a) What is the pressure at B?
(b) What is the temperature at C?
(c) Find the work done by the gas in one cycle and the thermodynamic efficiency of thiscycle.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry
Question Posted:





