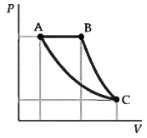
Two moles of a diatomic gas are taken through the cycle ABCA as shown on the PV
Question:
Two moles of a diatomic gas are taken through the cycle ABCA as shown on the PV diagram in figure. At A the pressure and temperature are 5 atm and 600 K. The volume at B is twice that at A. The segment BC is an adiabatic expansion and the segment CA is an isothermal compression.
(a) What is the volume of the gas at A?
(b) What are the volume and temperature of the gas at B?
(c) What is the temperature of the gas at C?
(d) What is the volume of the gas at C?
(e) How much work is done by the gas in each of the three segments of the cycle?
(f) How much heat is absorbed by the gas in each segment of the cycle?
(g) What is the thermodynamic efficiency of this cycle?
Step by Step Answer:

Fundamentals of Ethics for Scientists and Engineers
ISBN: 978-0195134889
1st Edition
Authors: Edmund G. Seebauer, Robert L. Barry





