Question: What cathode potential (versus S.H.E.) is required to reduce 99.99% of Cd(II) from a solution containing 0.10M Cd(II) in 1.0 M ammonia if there is
What cathode potential (versus S.H.E.) is required to reduce 99.99% of Cd(II) from a solution containing 0.10M Cd(II) in 1.0 M ammonia if there is negligible current? Consider the following reactions and assume that nearly all Cd(II) is in the form Cd(NH3)42+.
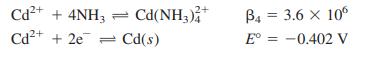
Cd2+ + 4NH3 Cd(NH,)* 2+ B4 = 3.6 x 10 Cd2+ + 2e = Cd(s) E = -0.402 V
Step by Step Solution
3.49 Rating (169 Votes )
There are 3 Steps involved in it
When 9999 of CdII is reduced the form... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
878-E-C-E-E-C (2111).docx
120 KBs Word File


