Question: What is the hybridization at each C in this molecule? Indicate the type of bond and the orbital's that are overlapping to form it for
What is the hybridization at each C in this molecule? Indicate the type of bond and the orbital's that are overlapping to form it for each of the designated bonds?
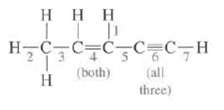
ITT H=C=C=C=C_C7H (both) H tall three)
Step by Step Solution
3.37 Rating (178 Votes )
There are 3 Steps involved in it
1 OCsp2 H1s 5 OCsp2 Cs... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-C (100).docx
120 KBs Word File


