Question: A slurry contains crystals of copper sulfate pentahydrate [CuSO 4 5H 2 O(s), specific gravity = 2.3] suspended in an aqueous copper sulfate solution
A slurry contains crystals of copper sulfate pentahydrate [CuSO4 ∙ 5H2O(s), specific gravity = 2.3] suspended in an aqueous copper sulfate solution (liquid SG = 1.2). A sensitive transducer is used to measure the pressure difference, ΔP (Pa), between two points in the sample container separated by a vertical distance of h meters. The reading is in turn used to determine the mass fraction of crystals in the slurry, x(kg crystals/kg slurry).
(a) Derive an expression for the transducer reading, P(Pa), in terms of the overall slurry density, p51(kg/m3), assuming that the pressure head formula of Chapter 3 (P P0 + pgh) is valid for this two-phase system.
(b) Validate the following expression relating the overall slurry density to the liquid and solid crystal densities (p and Pc) and the mass fraction of crystals in the slurry: (Suggestion: Insert units for all variables.)
(c) Suppose 175 kg of the slurry is placed in the sample container with h = 0.200 m and a transducer reading ΔP = 2775 Pa is obtained. Calculate (i) psi, (ii) xc, (iii) the total slurry volume, (iv) the mass of crystals in the slurry, (v) the mass of anhydrous copper sulfate (CuSO4 without the water of hydration) in the crystals. (vi) the mass of liquid solution, and (vii) the volume of liquid solution.
(d) Write a spreadsheet program to generate a calibration curve of xc versus ΔP for this device. Take as inputs pc (kg/m3), p1 (kg/m3), and h(m), arid calculate ΔP (Pa) for xc = 0.0.0.05,0.10,..., 0.60. Run the program for the parameter values in this problem (Pc = 2300. p = 1200, and h = 0.200). Then plot xc versus ΔP (have the spreadsheet program do it, if possible), and verify that the value of x corresponding to P = 2775 Pa on the calibration curve corresponds to the value calculated in part (c).
(e) Derive the expression in part (b). Take a basis of 1 kg of slurry [xc (kg), Vc (m3) crystals. (1 – xc) (kg), V1 (m3) liquid], and use the fact that the volumes of the crystals and liquid are additive.
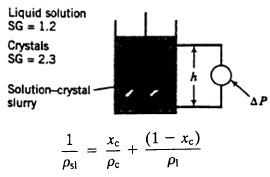
Liquid solution SG = 1.2 Crystals SG = 2.3 Solution-crystal- slurry AP (1 xe) Xe Psl Pc
Step by Step Solution
3.51 Rating (161 Votes )
There are 3 Steps involved in it
P Po Pagh a P PPagh AP P P P g hm hh h b 1Xc Pa ii Pc P 1 c i Pal P1 kg slurryL slurry kg slurry L s... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (2 attachments)
13-E-C-E-C-P (174).pdf
180 KBs PDF File
13-E-C-E-C-P (174).docx
120 KBs Word File


