Question: A thin-wall cylindrical vessel (1 m in diameter) is filled to a depth of 1.2 m with water at an initial temperature of 15?C. The
A thin-wall cylindrical vessel (1 m in diameter) is filled to a depth of 1.2 m with water at an initial temperature of 15?C. The water is well stirred by a mechanical agitator. Estimate the time required to heat the water to 50?C if the tank is suddenly immersed into oil at 105?C. The overall heat transfer coefficient between the oil and the water is 284 W/(m2 K), and the effective heat transfer surface area is 4.2 m2.GIVENA thin wall cylindrical vessel filled with water is suddenly immersed into oilDiameter of vessel (D) = 1 mDepth of water is vessel = 1.2 mInitial temperature (To) = 15?CFinal temperature (Tf) = 50?COil temperature (T??) = 105?CThe overall heat transfer coefficient between the oil and water (h) = 284 W/(m2 K)The effective heat transfer surface area (A) = 4.2 m2ASSUMPTIONSThe thermal capacitance of the cylindrical vessel is negligibleThe temperature of the water is uniformThe oil temperature remainsconstant
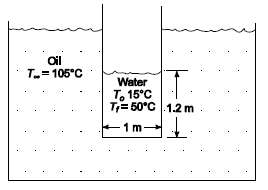
Oil T.=105C Water T. 15C T= 50C 1.2 m
Step by Step Solution
3.45 Rating (152 Votes )
There are 3 Steps involved in it
From Equation 283 ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
66-E-M-E-H-M-T (1536).docx
120 KBs Word File


