An adult takes about 12 breaths per minute inhaling roughly 500mL of air with each breath. The
Question:
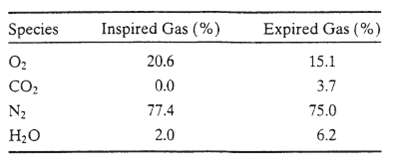
An adult takes about 12 breaths per minute inhaling roughly 500mL of air with each breath. The molar compositions of the inspired and expired gases are as follows: The inspired gas is at 24?C and 1 atm, and the expired gas is at body temperature and pressure?37?C and 1 atm. Nitrogen is not transported into or out of the blood in the lungs, so that (N2)in = (N2)out.
(a) Calculate the masses of O2, CO2, and H2O transferred from the pulmonary gases to the blood or vice versa (specify which) per minute.
(b) Calculate the volume of air exhaled per milliliter inhaled.
Fantastic news! We've Found the answer you've been seeking!
Step by Step Answer:
Related Book For 

Elementary Principles of Chemical Processes
ISBN: 978-0471720638
3rd Edition
Authors: Richard M. Felder, Ronald W. Rousseau
Question Posted:





