Question: An unknown compound has the formula C6H10. (a) What is the DU for this compound? (b) When a solution of Br2 in CC14 is added
An unknown compound has the formula C6H10.
(a) What is the DU for this compound?
(b) When a solution of Br2 in CC14 is added to the unknown, the bromine color disappears. What information does this provide about the structure of the unknown?
(c) The unknown reacts with excess H2 in the presence of Pt to give C6H12. What information dos these provide about the structure of the unknown?
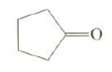
(d) This unknown is one of the products isolated from the ozonolysis of the unknown. What is the structure of theunknown?
Step by Step Solution
3.43 Rating (172 Votes )
There are 3 Steps involved in it
a The DU for this compound is 2 b The disappearance of the bromine color is an ... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-C (182).docx
120 KBs Word File


