Question: Carboxylic acids having a second carbonyl group two atoms away lose CO2 (decarboxylate.) through an intermediate enolate ion when treated with base. Write the mechanism
Carboxylic acids having a second carbonyl group two atoms away lose CO2 (decarboxylate.) through an intermediate enolate ion when treated with base. Write the mechanism of this decarboxylation reaction using curved arrows to show the electron flow in eachstep.
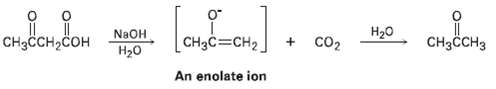
|| CHH, H20 CO2 NaOH CHCCH CH3C=CH2 2 An enolate ion
Step by Step Solution
★★★★★
3.39 Rating (165 Votes )
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock

OH HH deproton ation H3C loss o... View full answer

Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock
Document Format (1 attachment)
22-C-O-CA (97).docx
120 KBs Word File


