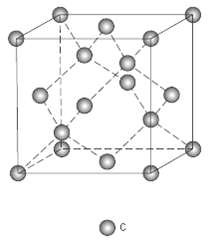
Question: Compute the atomic packing factor for the diamond cubic crystal structure (Figure 12.15). Assume that bonding atoms touch one another, that the angle between adjacent
Compute the atomic packing factor for the diamond cubic crystal structure (Figure 12.15). Assume that bonding atoms touch one another, that the angle between adjacent bonds is 109.5°, and that each atom internal to the unit cell is positioned a/4 of the distance away from the two nearest cell faces (a is the unit cell edgelength).
O C
Step by Step Solution
3.50 Rating (170 Votes )
There are 3 Steps involved in it
We are asked in this problem to compute the atomic packing factor for the diamond c... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
33-E-M-S-E-M-S (481).docx
120 KBs Word File


