Question: Draw both chair conformations for menthol (a component of peppermint oil) and its stereo isomer, neo menthol. Which groups are axial and which groups are
Draw both chair conformations for menthol (a component of peppermint oil) and its stereo isomer, neo menthol. Which groups are axial and which groups are equatorial? Explain which conformation is more stable for each stereo isomer. Which stereo isomer is more stable by how much energy?
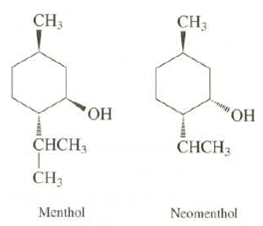
CH3 CH3 " " CH CH, CH3 Menthol Neomenthol
Step by Step Solution
3.52 Rating (162 Votes )
There are 3 Steps involved in it
For menthol the groups are either all axial or all equatorial Obviously th... View full answer

Get step-by-step solutions from verified subject matter experts
Document Format (1 attachment)
15-C-O-S (71).docx
120 KBs Word File


