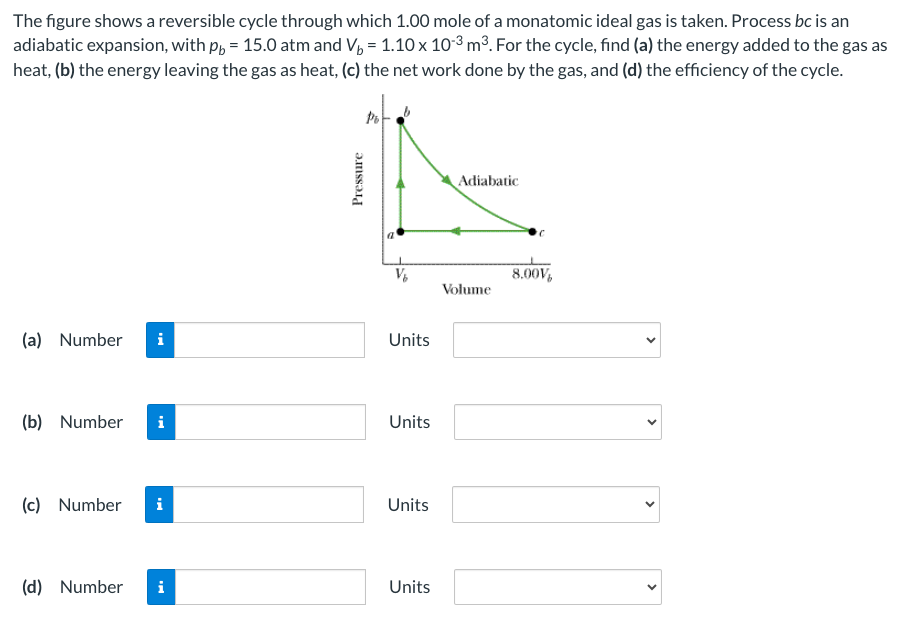
Question: The figure shows a reversible cycle through which 1 . 0 0 mole of a monatomic ideal gas is taken. Process b c is an
The figure shows a reversible cycle through which mole of a monatomic ideal gas is taken. Process is an
adiabatic expansion, with atm and For the cycle, find a the energy added to the gas as
heat, b the energy leaving the gas as heat, c the net work done by the gas, and d the efficiency of the cycle.
a Number
Units
b Number
Units
c Number
Units
d Number
Units
Step by Step Solution
There are 3 Steps involved in it
1 Expert Approved Answer
Step: 1 Unlock


Question Has Been Solved by an Expert!
Get step-by-step solutions from verified subject matter experts
Step: 2 Unlock
Step: 3 Unlock


